Abstract
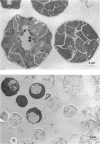
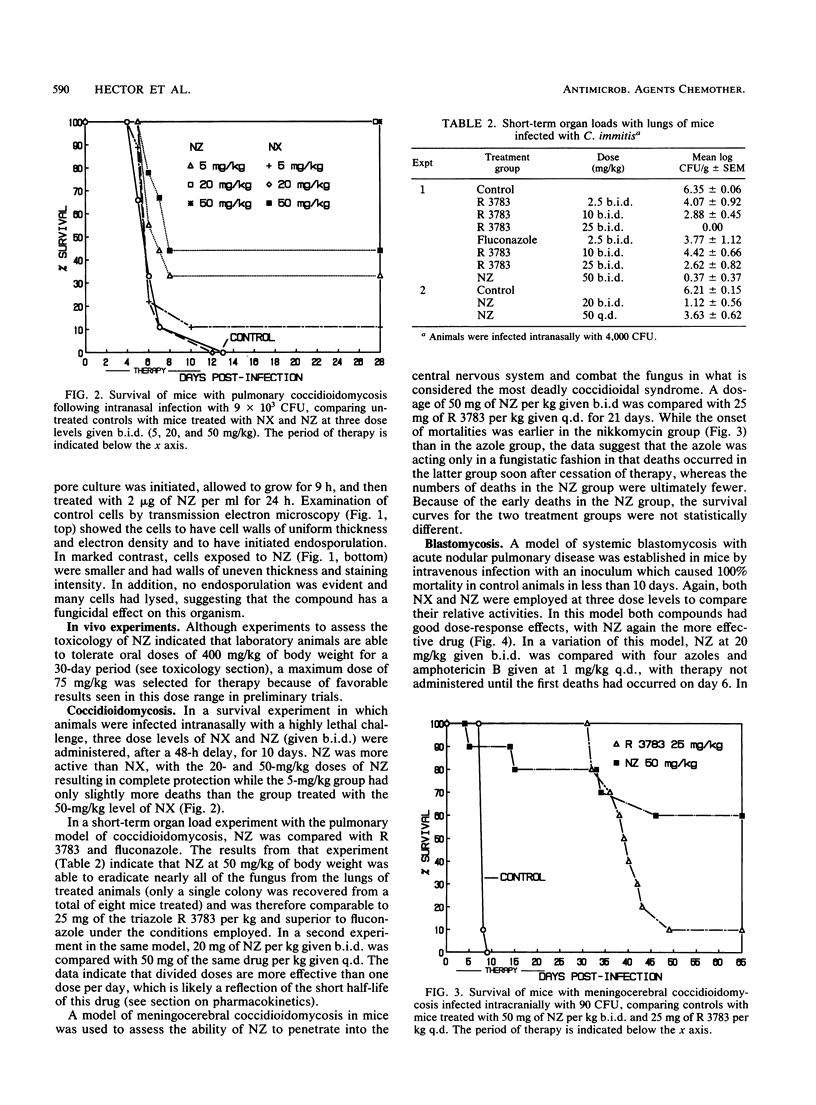
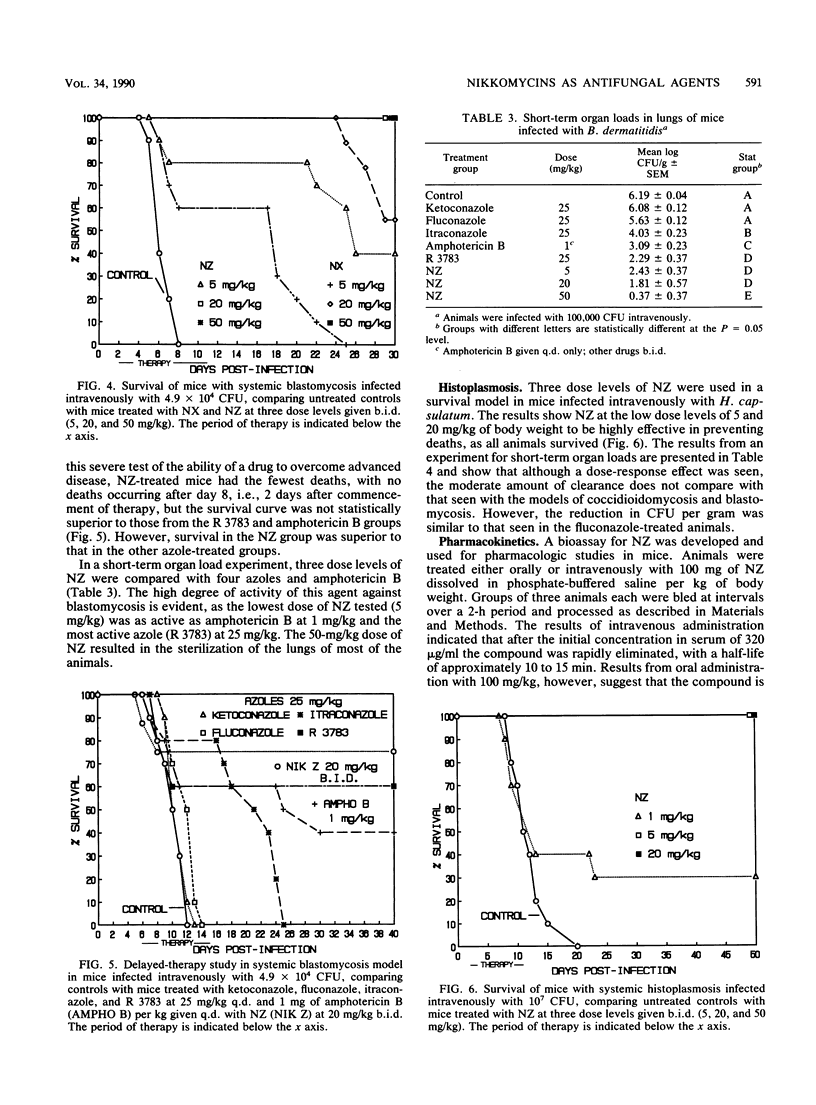
Nikkomycins X and Z, competitive inhibitors of fungal chitin synthase, were evaluated as therapeutic agents in vitro and in mouse models of coccidioidomycosis, histoplasmosis, and blastomycosis. In vitro, the nikkomycins were found to be most effective against the highly chitinous, dimorphic fungi Coccidioides immitis and Blastomyces dermatitidis, were less effective against yeasts, and were virtually without effect on the filamentous fungus Aspergillus fumigatus. Additionally, by transmission electron microscopy, nikkomycin Z was highly disruptive to the cell wall and internal structure of the spherule-endospore phase of C. immitis in vitro. In vivo, nikkomycin Z was more effective than nikkomycin X, was also found to be superior on a milligram per milligram basis to the majority of azoles tested in the models of coccidioidomycosis and blastomycosis, and was moderately effective in histoplasmosis. A study of the pharmacokinetics in mice showed that nikkomycin Z was rapidly eliminated after intravenous infusion but that absorption after oral administration was sufficiently slow to allow inhibitory levels to persist for more than 2 h. Results of limited toxicology tests suggest that nikkomycin Z was well tolerated at the dosages employed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. M., Covert N. L., Shenbagamurthi P., Steinfeld A. S., Naider F. Polyoxin D inhibits growth of zoopathogenic fungi. Antimicrob Agents Chemother. 1983 Jun;23(6):926–929. doi: 10.1128/aac.23.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun P. C., Calderone R. A. Chitin synthesis in Candida albicans: comparison of yeast and hyphal forms. J Bacteriol. 1978 Mar;133(3):1472–1477. doi: 10.1128/jb.133.3.1472-1477.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillinger G. U. Metabolic products of microorganisms. 181. Chitin synthase from fungi, a test model for substances with insecticidal properties. Arch Microbiol. 1979 Apr;121(1):71–74. doi: 10.1007/BF00409207. [DOI] [PubMed] [Google Scholar]
- Cabib E. The synthesis and degradation of chitin. Adv Enzymol Relat Areas Mol Biol. 1987;59:59–101. doi: 10.1002/9780470123058.ch2. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- Davis T. E., Jr, Domer J. E., Li Y. T. Cell wall studies of Histoplasma capsulatum and Blastomyces dermatitidis using autologous and heterologous enzymes. Infect Immun. 1977 Mar;15(3):978–987. doi: 10.1128/iai.15.3.978-987.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzer J., Fiedler H. P., Müller H., Zähner H., Rathmann R., Ernst K., König W. A. New nikkomycins by mutasynthesis and directed fermentation. J Antibiot (Tokyo) 1984 Jan;37(1):80–82. doi: 10.7164/antibiotics.37.80. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Hamilton J. G., Harkin J. C. Comparative study of the cell walls of the yeastlike and mycelial phases of Histoplasma capsulatum. J Bacteriol. 1967 Aug;94(2):466–474. doi: 10.1128/jb.94.2.466-474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J Bacteriol. 1971 Sep;107(3):870–877. doi: 10.1128/jb.107.3.870-877.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila T., San-Blas G., San-Blas F. Effect of papulacandin B on glucan synthesis in Paracoccidioides brasiliensis. J Med Vet Mycol. 1986 Jun;24(3):193–202. [PubMed] [Google Scholar]
- Dähn U., Hagenmaier H., Höhne H., König W. A., Wolf G., Zähner H. Stoffwechselprodukte von mikroorganismen. 154. Mitteilung. Nikkomycin, ein neuer hemmstoff der chitinsynthese bei pilzen. Arch Microbiol. 1976 Mar 19;107(2):143–160. doi: 10.1007/BF00446834. [DOI] [PubMed] [Google Scholar]
- Farkas V. Biosynthesis of cell walls of fungi. Microbiol Rev. 1979 Jun;43(2):117–144. doi: 10.1128/mr.43.2.117-144.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooday B. W. Biosynthesis of the fungal wall - mechanisms and implications. The first Fleming Lecture. J Gen Microbiol. 1977 Mar;99(1):1–11. doi: 10.1099/00221287-99-1-1. [DOI] [PubMed] [Google Scholar]
- Hector R. F., Pappagianis D. Inhibition of chitin synthesis in the cell wall of Coccidioides immitis by polyoxin D. J Bacteriol. 1983 Apr;154(1):488–498. doi: 10.1128/jb.154.1.488-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector R. F., Zimmer B. L., Pappagianis D. Microtiter method for MIC testing with spherule-endospore-phase Coccidioides immitis. J Clin Microbiol. 1988 Dec;26(12):2667–2668. doi: 10.1128/jcm.26.12.2667-2668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Gil F., Azuma I. Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma capsulatum. Mycopathol Mycol Appl. 1974 Oct 15;54(1):1–13. doi: 10.1007/BF02055967. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- McCarthy P. J., Troke P. F., Gull K. Mechanism of action of nikkomycin and the peptide transport system of Candida albicans. J Gen Microbiol. 1985 Apr;131(4):775–780. doi: 10.1099/00221287-131-4-775. [DOI] [PubMed] [Google Scholar]
- Pollack J. H., Lange C. F., Hashimoto T. "Nonfibrillar" chitin associated with walls and septa of Trichophyton mentagrophytes arthrospores. J Bacteriol. 1983 May;154(2):965–975. doi: 10.1128/jb.154.2.965-975.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. A., Jaworski E. G. The synthesis of chitin by particulate preparations of Allomyces macrogynus. Biochemistry. 1966 Apr;5(4):1149–1154. doi: 10.1021/bi00868a006. [DOI] [PubMed] [Google Scholar]
- Reiss E. Serial enzymatic hydrolysis of cell walls of two serotypes of yeast-form Histoplasma capsulatum with alpha(1 leads to 3)-glucanase, beta(1 leads to 3)-glucanase, pronase, and chitinase. Infect Immun. 1977 Apr;16(1):181–188. doi: 10.1128/iai.16.1.181-188.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Poulain D., Herbaut J., Biguet J. Localization of chitin in the cell wall of Candida albicans by means of wheat germ agglutinin. Fluorescence and ultrastructural studies. Eur J Cell Biol. 1981 Dec;26(1):121–128. [PubMed] [Google Scholar]
- Wheat R. W., Tritschler C., Conant N. F., Lowe E. P. Comparison of Coccidioides immitis arthrospore, mycelium, and spherule cell walls, and influence of growth medium on mycelial cell wall composition. Infect Immun. 1977 Jul;17(1):91–97. doi: 10.1128/iai.17.1.91-97.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadan J. C., Gonneau M., Sarthou P., Le Goffic F. Sensitivity to nikkomycin Z in Candida albicans: role of peptide permeases. J Bacteriol. 1984 Dec;160(3):884–888. doi: 10.1128/jb.160.3.884-888.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]