Abstract
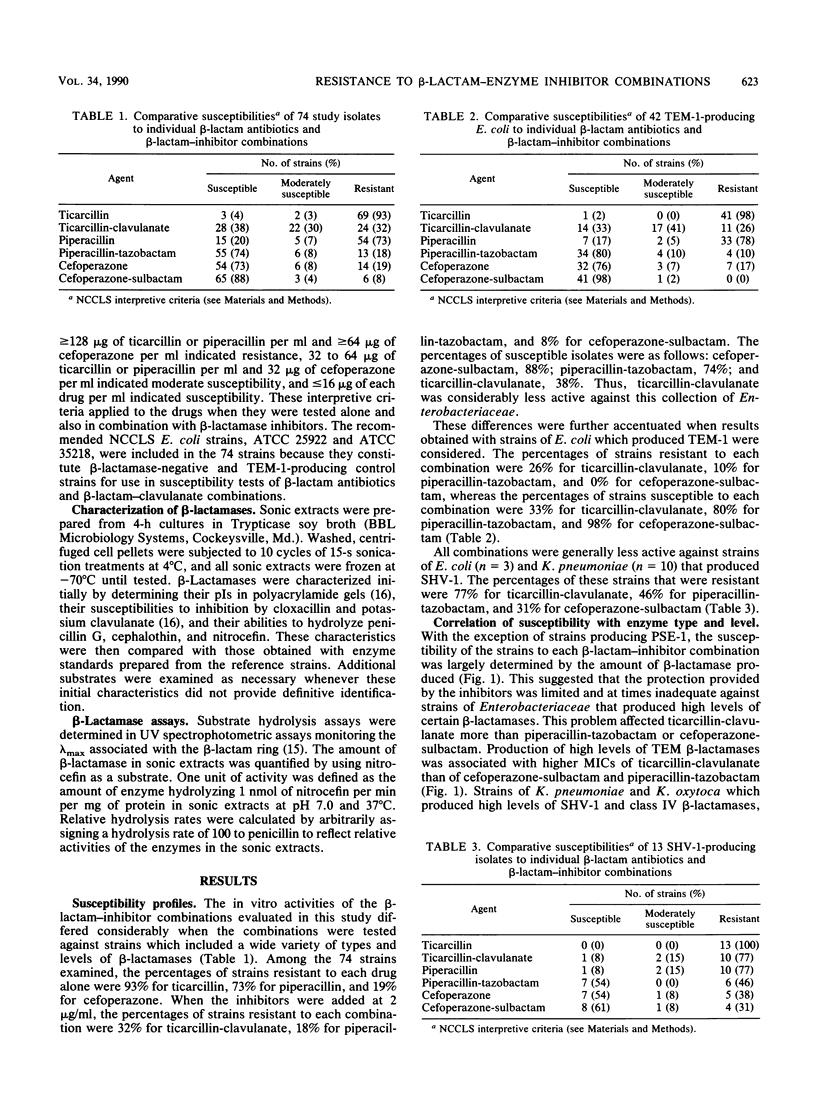
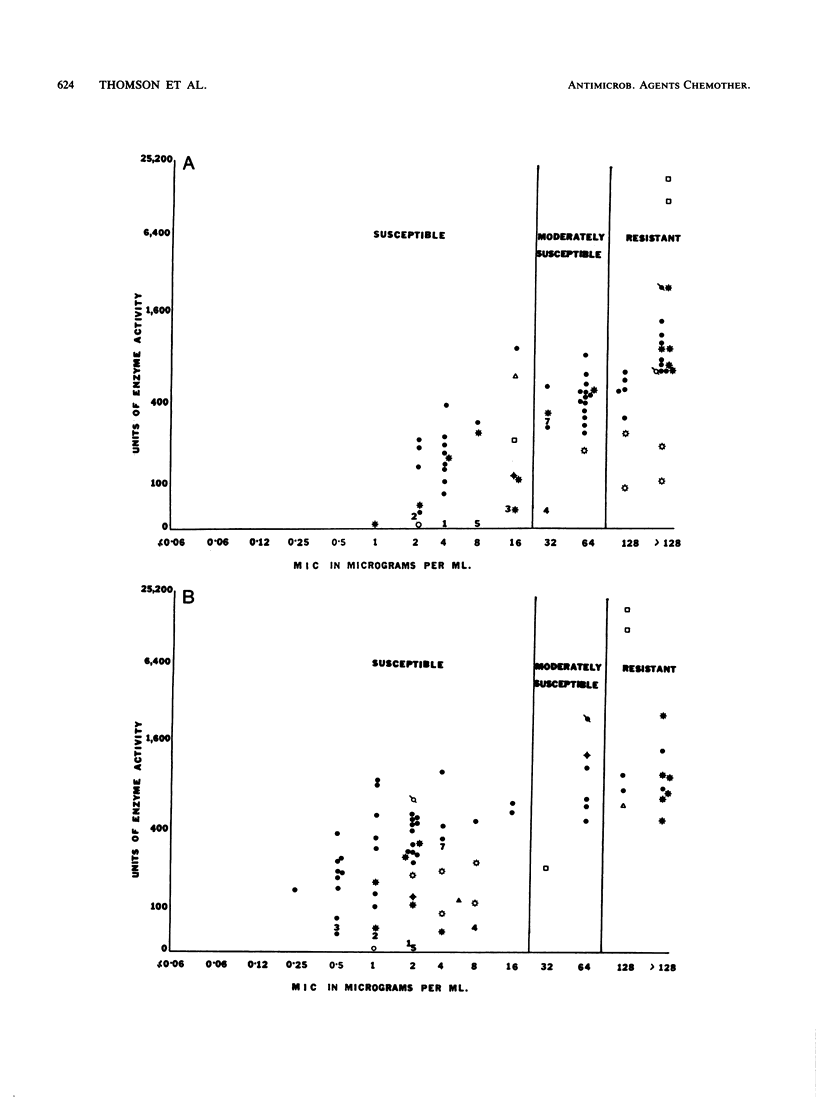
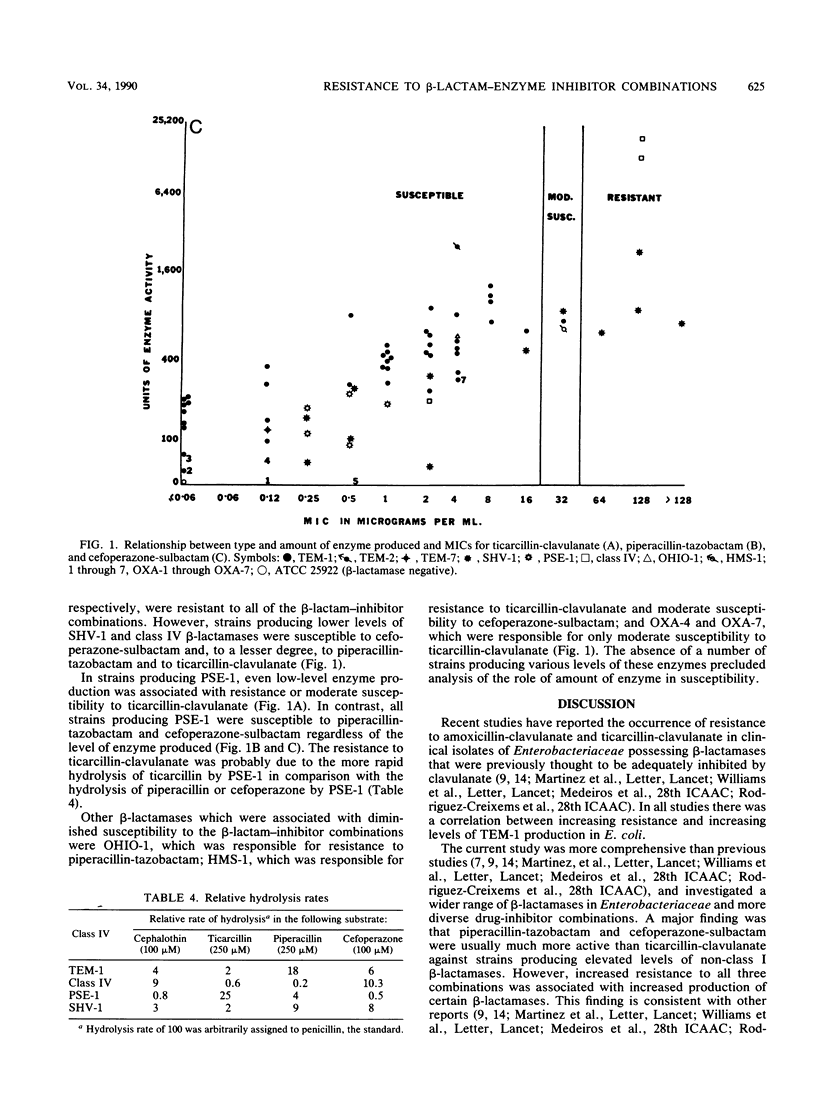
Recent reports that members of the family Enterobacteriaceae that produce high levels of certain beta-lactamases are often resistant to ticarcillin-clavulanate prompted this study to assess the relationship between type and amount of enzyme produced and susceptibility to ticarcillin-clavulanate, piperacillin-tazobactam, and cefoperazone-sulbactam. Agar dilution MICs were determined by using 73 strains of Enterobacteriaceae that produced a single beta-lactamase that had been characterized and quantified and a beta-lactamase-negative control strain of Escherichia coli. For E. coli and Klebsiella pneumoniae, MICs of each combination increased as levels of TEM, SHV-1, or class IV enzymes increased. However, the percentage of strains that were resistant was highest for ticarcillin-clavulanate (32%), with only 18 and 6% resistant to piperacillin-tazobactam and cefoperazone-sulbactam, respectively. Strains producing PSE-1, regardless of level, were resistant or moderately susceptible to ticarcillin-clavulanate but were susceptible to piperacillin-tazobactam and cefoperazone-sulbactam. HMS-1 and OHIO-1 beta-lactamases were associated with resistance to ticarcillin-clavulanate and piperacillin-tazobactam, respectively. High levels of class IV enzymes in Klebsiella oxytoca were associated with resistance to all three combinations. These results indicate that the level and type of beta-lactamase produced by members of the family Enterobacteriaceae are important determinants of susceptibility to beta-lactam-inhibitor combinations, especially ticarcillin-clavulanate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acar J. F., Gutmann L., Kitzis M. D. Beta-lactamases in clinical isolates. Spectrum implications of sulbactam/ampicillin. Drugs. 1988;35 (Suppl 7):12–16. doi: 10.2165/00003495-198800357-00004. [DOI] [PubMed] [Google Scholar]
- Aldridge K. E., Sanders C. V., Marier R. L. Variation in the potentiation of beta-lactam antibiotic activity by clavulanic acid and sulbactam against multiply antibiotic-resistant bacteria. J Antimicrob Chemother. 1986 Apr;17(4):463–469. doi: 10.1093/jac/17.4.463. [DOI] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 1, 2a, 2b, and 2b'. Antimicrob Agents Chemother. 1989 Mar;33(3):264–270. doi: 10.1128/aac.33.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. M., Zemcov S. J. Clavulanic acid in combination with ticarcillin: an in-vitro comparison with other beta-lactams. J Antimicrob Chemother. 1984 Feb;13(2):121–128. doi: 10.1093/jac/13.2.121. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Kitzis M. D., Yamabe S., Acar J. F. Comparative evaluation of a new beta-lactamase inhibitor, YTR 830, combined with different beta-lactam antibiotics against bacteria harboring known beta-lactamases. Antimicrob Agents Chemother. 1986 May;29(5):955–957. doi: 10.1128/aac.29.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. R., Aronoff S. C., Johenning S., Yamabe S. Comparative activities of the beta-lactamase inhibitors YTR 830, clavulanate and sulbactam combined with extended-spectrum penicillins against ticarcillin-resistant Enterobacteriaceae and pseudomonads. J Antimicrob Chemother. 1986 Aug;18(2):177–184. doi: 10.1093/jac/18.2.177. [DOI] [PubMed] [Google Scholar]
- Marre R., Schulz E. In vitro activity of mecillinam and amoxicillin/clavulanic acid against strains of Escherichia coli producing TEM-1, Oxa-1 and chromosomal beta-lactamases. Arzneimittelforschung. 1988 Jul;38(7):863–865. [PubMed] [Google Scholar]
- Medeiros A. A. Beta-lactamases. Br Med Bull. 1984 Jan;40(1):18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., Hedges R. W., Jacoby G. A. Spread of a "Pseudomonas-specific" beta-lactamase to plasmids of enterobacteria. J Bacteriol. 1982 Feb;149(2):700–707. doi: 10.1128/jb.149.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Iaconis J. P., Bodey G. P., Samonis G. Resistance to ticarcillin-potassium clavulanate among clinical isolates of the family Enterobacteriaceae: role of PSE-1 beta-lactamase and high levels of TEM-1 and SHV-1 and problems with false susceptibility in disk diffusion tests. Antimicrob Agents Chemother. 1988 Sep;32(9):1365–1369. doi: 10.1128/aac.32.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Moland E. S. Characterization of beta-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986 Dec;30(6):951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]


