Tumors of odontogenic origin (arising in tissues which give origin to the teeth) are the most common benign tumors of the canine oral cavity (1). ‘Epulis’ is a nonspecific term that refers to tumors and tumor-like masses of the gingiva. Three types of odontogenic tumors have been described in the veterinary literature: fibromatous epulis, ossifying epulis, and acanthomatous epulis (2). While fibromatous and ossifying epulides are confined to the gingiva, acanthomatous epulides often extensively invade adjacent bone (3). Recently, the term canine acanthomatous ameloblastoma has been recommended to describe acanthomatous epulis in the dog (3). “Ameloblastoma” relates to the cell type of origin, odontogenic epithelial cells, and “acanthomatous” refers to the spiny shape of epithelial cells within these tumors (3).
Canine acanthomatous ameloblastoma presents as an exophytic, irregular gingival mass on either side of the dental arcade, with a predilection for the rostral region of the mandible (Figure 1) (3). Involvement of the underlying bone is common (Figure 2). Once a histological diagnosis is known, the primary tumor should be staged using the World Health Organization (WHO) Classification System for tumors of the oral cavity (Table 1) (4). Metastasis to regional lymph nodes or other distant organs has not been reported (5). Computed tomography or magnetic resonance imaging is often recommended prior to surgical or radiation treatment to accurately determine the extent of the primary tumor (5). Magnetic resonance images have been reported to provide more accurate assessment of oral tumor margins in soft tissue and bone than computed tomographic images (6).
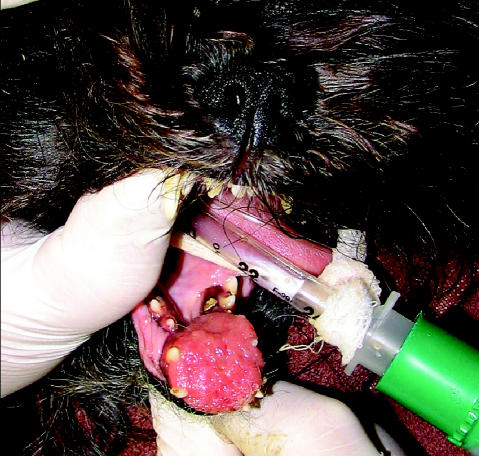
Figure 1.
A canine acanthomatous ameloblastoma located in the rostral mandible, displacing adjacent teeth.
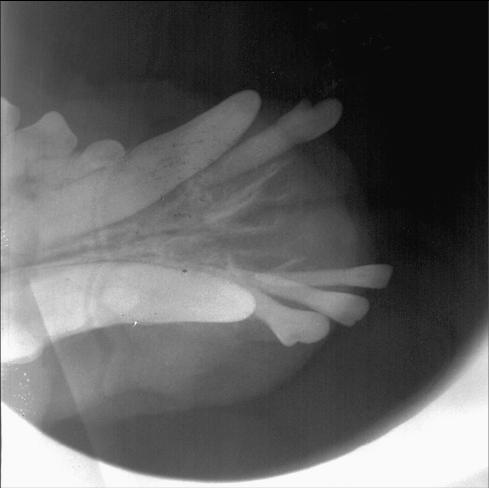
Figure 2.
Dental radiograph of the patient in Figure 1 showing bone lysis and loss of teeth adjacent to the tumor.
Table 1.
Clinical T stage of canine tumors of the oral cavitya
| T0 | No evidence of tumor | |
| T1 | Tumor < 2 cm maximum diameter
T1a without bone invasion |
T1b with bone invasion |
| T2 | Tumor 2 to 4 cm maximum diameter
T2a without bone invasion |
T2b with bone invasion |
| T3 | Tumor > 4 cm maximum diameter
T3a without bone invasion |
T3b with bone invasion |
Tis category not included here
In contrast to fibromatous and ossifying epulides, local recurrence of canine acanthomatous ameloblastoma is common after conservative local excision (5,7,8). Due to the highly infiltrative behavior of this tumor, wide local excision of adjacent soft tissue and bone is necessary for cure, and is the treatment of choice (5,7). In the author’s experience, a minimum of 2 cm margins to gross or radiographically detectable disease is recommended. Radiation therapy is indicated for dogs with tumors that are not curable with surgery alone. It may not be possible to remove all tumor cells surgically due to the location or size of a tumor, or due to the desire to preserve function or cosmesis. A prospective clinical trial of 47 dogs with acanthomatous epulides (39 dogs) or fibromatous or ossifying epulides (8 dogs) found that radiation therapy was a safe and effective treatment for WHO stage T1 (< 2 cm maximum diameter) and T2 tumors (2–4 cm maximum diameter) (9). Ten dogs had WHO stage T1 tumors, 30 had stage T2 tumors, and 7 had stage T3 tumors. Thirty-eight dogs had bone involvement. The prescribed radiation dose was 12 fractions of 4 Gy 3 times a week to a total dose of 48 Gy. Anatomic site, tumor size, WHO T stage, and bone involvement were among the factors examined as prognostic indicators of progression-free survival rates. Progression-free survival was defined as the time between completion of radiation treatment and detection of measurable local tumor recurrence, or death from a cause unrelated to tumor, whichever came first. Clinical stage was the only significant prognostic factor (P = 0.0303), with dogs that had WHO stage T3 tumors having a 7.9 times higher risk of tumor recurrence than dogs with WHO stage T1 tumors. Dogs with stage T1 and T2 tumors had a similar risk of recurrence. The estimate of 3-year progression free survival for dogs with acanthomatous epulis was 80%. Tumor recurrence was diagnosed in 7 of the 39 dogs with acanthomatous epulis. The effect of tumor size on progression-free survival rate reported in this paper supports the need for early diagnosis and treatment of these tumors.
A 1984 study reported a median survival of 37 mo in 39 dogs with acanthomatous epulides treated with orthovoltage radiation (10). Cause of death in most dogs was not related to their acanthomatous epulis. Seven of the 39 dogs (18%) developed a malignant tumor type other than acanthomatous epulis at the site of the original tumor, at a median time of 47 mo after completion of radiation therapy. Five of the 7 new tumors were squamous cell carcinomas. The author termed the development of these new tumors “malignant transformation,” and suggested the possibility that radiation had induced the original benign acanthomatous epulides to transform into a malignant tumor type (10,11). However, in the report of a 2004 study of 57 dogs, which included dogs from a previously reported study (9), cowritten by the same author, no malignant epithelial tumors developed after treatment of acanthomatous epulis with radiation therapy (12). This latter study suggested that the transformations to squamous cell carcinomas reported in the 1984 paper may have been due to an incorrect initial diagnosis of squamous cell carcinoma as acanthomatous epulis, or to the limitations in treatment effectiveness when orthovoltage radiation therapy is used. The median overall survival for the 57 dogs for death due to any cause was 48 mo, and the authors concluded that radiation therapy is an effective treatment for acanthomatous epulis, with no increased risk of radiation-induced cancer over other tumor types.
Radiation treatment fields for canine acanthomatous ameloblastoma will include skin and oral mucosa, and acute side effects will develop in these tissues. Mucositis in the treated region of the oral cavity generally starts to develop during the 2nd wk of treatment, and is most severe during the 4th wk of treatment (13). Skin effects in the treatment field include epilation and dry to moist desquamation; they usually become apparent during the 3rd wk of treatment (Figure 3). Acute side effects generally subside 2–4 wk after completion of the radiation treatment (13). Skin hypopigmentation or hyperpigmentation and temporary or permanent hair loss in the treatment field will occur (Figure 4). Macroscopic tumors generally continue to decrease in size after completion of radiation treatment (Figure 5).
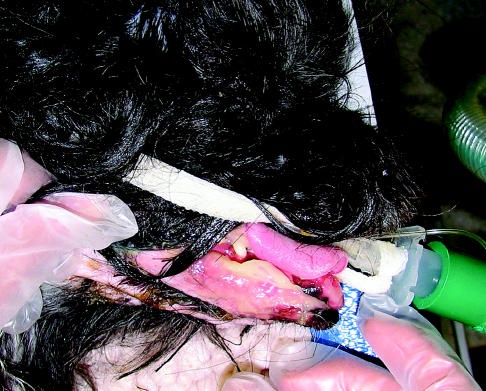
Figure 3.
Acute skin effects in the patient in Figure 1, 21 d after the start of radiation treatment, with moist desquamation of the skin in the radiation treatment field.
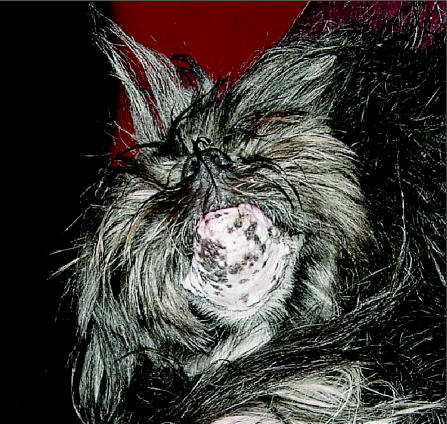
Figure 4.
The same patient 9 wk after completion of radiation therapy, with alopecia and skin pigmentation change in the treated area.
Figure 5.
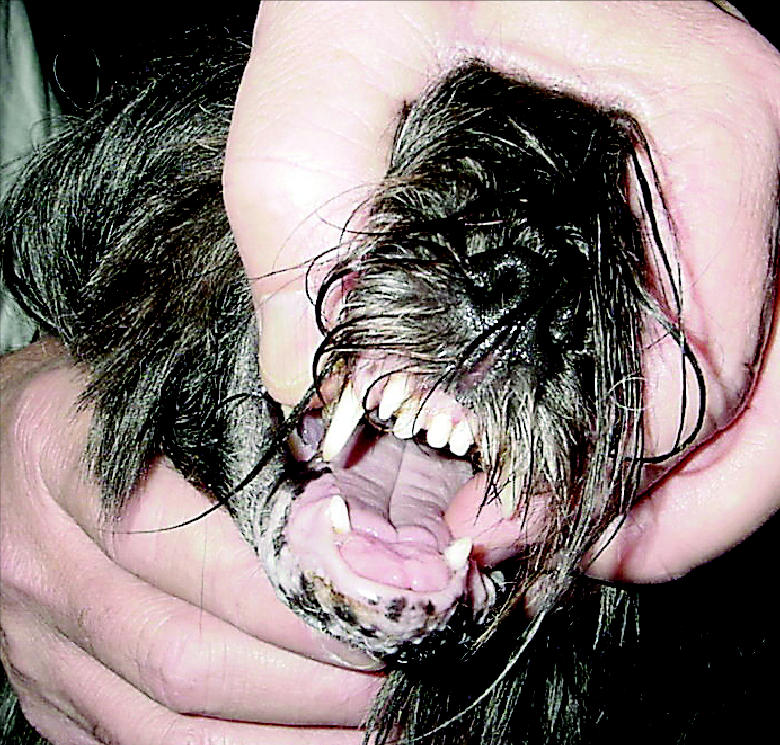
The tumor 9 wk after completion of radiation therapy.
Footnotes
The authors thank John and Shirley Clark for kindly providing the photographs of Nouggie.
References
- 1.Spodnick GJ, Page RL. Canine and feline oropharyngeal neoplasms. In: Kirk RW, ed. Kirk’s Current Veterinary Therapy, 8th edition, Philadelphia: WB Saunders, 1995:691–695.
- 2.Dubielzig RR, Goldschmidt MH, Brodey RS. The nomenclature of periodontal epulides in dogs. Vet Pathol. 1979;16:209–214. doi: 10.1177/030098587901600206. [DOI] [PubMed] [Google Scholar]
- 3.Head KW, Else RW, Dubielzig RR. Tumors of the alimentary tract. In: Meuten DJ, ed. Tumors in Domestic Animals, 4th ed. Ames: Iowa State Univ Pr, 2002:401–481.
- 4.World Health Organization, TNM classification of tumors in domestic animals. Geneva, Switzerland, 1980:46–47.
- 5.Withrow SJ. Cancer of the gastrointestinal tract. In: Withrow SJ, MacEwen EG, eds. Small Animal Clinical Oncology. Philadelphia: WB Saunders, 2001:305–353.
- 6.Kafka UC, Carstens A, Steenkamp G, Symington H. Diagnostic value of magnetic resonance imaging and computed tomography for oral masses in dogs. J S Afr Vet Assoc. 2004;75:163–168. doi: 10.4102/jsava.v75i4.476. [DOI] [PubMed] [Google Scholar]
- 7.White RA, Gorman NT. Wide local excision of acanthomatous epulides in the dog. Vet Surg. 1989;18:12–14. doi: 10.1111/j.1532-950x.1989.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 8.Bjorling DE, Chambers JN, Mahaffey EA. Surgical treatment of epulides in dogs: 25 cases (1974–1984) J Am Vet Med Assoc. 1987;190:1315–1318. [PubMed] [Google Scholar]
- 9.Theon AP, Rodriguez C, Griffey S, Madewell BR. Analysis of prognostic factors and patterns of failure in dogs with periodontal tumors treated with megavoltage irradiation. J Am Vet Med Assoc. 1997;210:785–788. [PubMed] [Google Scholar]
- 10.Thrall DE. Orthovoltage radiotherapy of acanthomatous epulides in 39 dogs. J Am Vet Med Assoc. 1984;184:826–829. [PubMed] [Google Scholar]
- 11.Thrall DE, Goldschmidt MH, Biery DN. Malignant tumor formation at the site of previously irradiated acanthomatous epulides in four dogs. J Am Vet Med Assoc. 1981;178:127–132. [PubMed] [Google Scholar]
- 12.McEntee MC, Page RL, Theon A, Erb HN, Thrall DE. Malignant tumor formation in dogs previously irradiated for acanthomatous epulis. Vet Radiol Ultrasound. 2004;45:357–361. doi: 10.1111/j.1740-8261.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 13.Gillette EL, LaRue SM, Gillette SM. Normal tissue tolerance and management of radiation injury. Semin Vet Med Surg (Small Anim) 1995;10:209–213. [PubMed] [Google Scholar]