Abstract
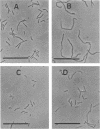
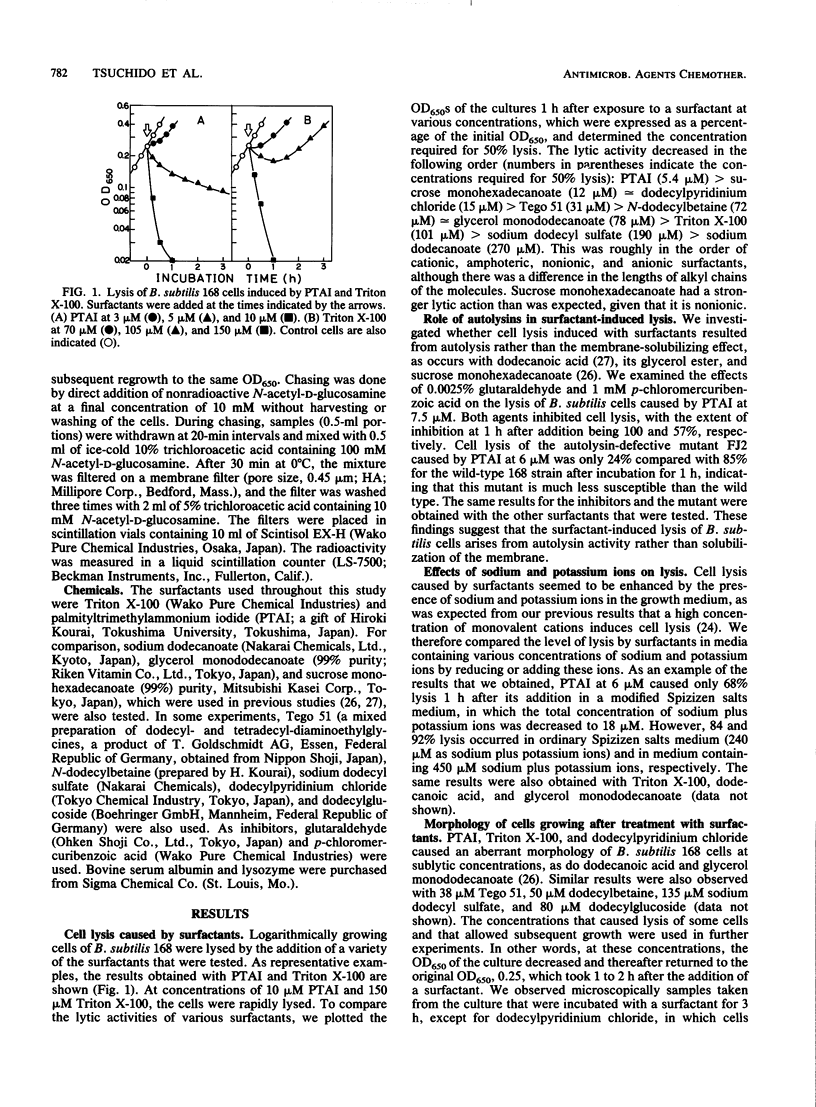
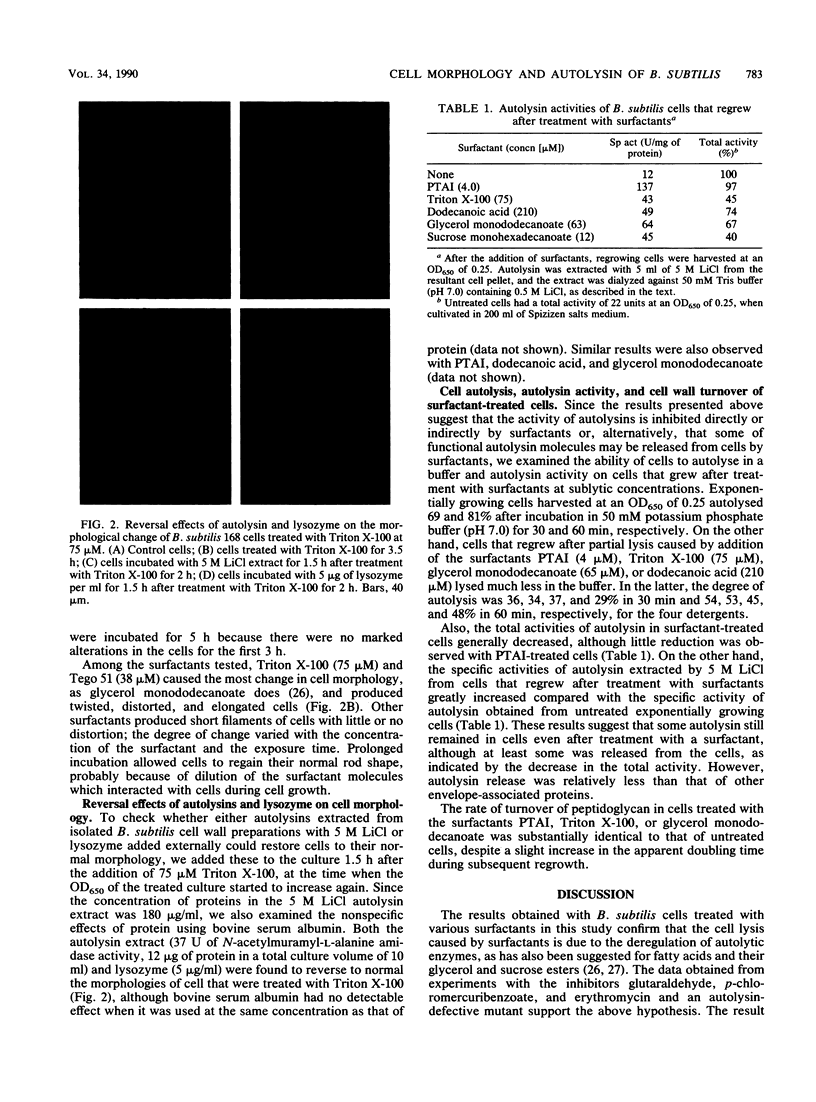
The surfactants tested in this study lysed Bacillus subtilis 168 cells at the logarithmic growth phase. Results obtained with inhibitors and a mutant that had defective autolytic enzymes suggested that cell lysis resulted from the deregulation of autolysin activity. The addition of surfactants at sublytic concentrations produced twisted cells, filamented cells, or both. Autolysins extracted with 5 M LiCl from the cell wall fraction and lysozyme added to cells that were treated with surfactants restored the apparently normal cell rod morphology, suggesting that surfactants interfere with the role of autolysins in normal construction of the cell envelope. The rates of cellular autolysis and autolysin activity remaining in growing cells after exposure to a surfactant at a sublytic concentration decreased, although the rate of turnover of cell wall peptidoglycan was the same as that of control cells. Surfactants were suggested to interact with the regulatory system of autolysins and, thus, to affect the activities of autolysins in B. subtilis cells and to cause either morphological changes or cell autolysis, depending on the concentration of surfactants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung H. Y., Freese E. Monovalent cations enable cell wall turnover of the turnover-deficient lyt-15 mutant of Bacillus subtilis. J Bacteriol. 1985 Mar;161(3):1222–1225. doi: 10.1128/jb.161.3.1222-1225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Shockman G. D. Cellular lysis of Streptococcus faecalis induced with triton X-100. J Bacteriol. 1978 Jul;135(1):153–160. doi: 10.1128/jb.135.1.153-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON I. M., LOMINSKI I., STERN H. An electron-microscope study of the action of cetyl-trimethyl-ammonium bromide on Staphylococcus aureus. J Pathol Bacteriol. 1953 Oct;66(2):513–526. doi: 10.1002/path.1700660223. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Koch A. L. The functions of autolysins in the growth and division of Bacillus subtilis. Crit Rev Microbiol. 1987;15(2):169–222. doi: 10.3109/10408418709104457. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. Mutant of Bacillus subtilis demonstrating the requirement of lysis for growth. J Bacteriol. 1971 Feb;105(2):629–636. doi: 10.1128/jb.105.2.629-636.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D., Mendelson N. H., Thwaites J. J. Relaxation motions induced in Bacillus subtilis macrofibres by cleavage of peptidoglycan. J Gen Microbiol. 1986 Aug;132(8):2377–2385. doi: 10.1099/00221287-132-8-2377. [DOI] [PubMed] [Google Scholar]
- Fein J. E. Possible involvement of bacterial autolytic enzymes in flagellar morphogenesis. J Bacteriol. 1979 Feb;137(2):933–946. doi: 10.1128/jb.137.2.933-946.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton P. J., Hobbs D. G. Penicillin and cell wall synthesis: a study of Bacillus licheniformis by electron microscopy. J Bacteriol. 1971 May;106(2):646–658. doi: 10.1128/jb.106.2.646-658.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Mechanism of lysis of Escherichia coli by ethanol and other chaotropic agents. J Bacteriol. 1981 Apr;146(1):331–336. doi: 10.1128/jb.146.1.331-336.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mendelson N. H. Bacterial growth and division: genes, structures, forces, and clocks. Microbiol Rev. 1982 Sep;46(3):341–375. doi: 10.1128/mr.46.3.341-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Favre D., Thwaites J. J. Twisted states of Bacillus subtilis macrofibers reflect structural states of the cell wall. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3562–3566. doi: 10.1073/pnas.81.11.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Raychaudhuri D., Chatterjee A. N. Use of resistant mutants to study the interaction of triton X-100 with Staphylococcus aureus. J Bacteriol. 1985 Dec;164(3):1337–1349. doi: 10.1128/jb.164.3.1337-1349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Taylor C., Rayter S., Ward J. B. Purification and properties of autolytic endo-beta-N-acetylglucosaminidase and the N-acetylmuramyl-L-alanine amidase from Bacillus subtilis strain 168. J Gen Microbiol. 1984 Sep;130(9):2395–2402. doi: 10.1099/00221287-130-9-2395. [DOI] [PubMed] [Google Scholar]
- Shohayeb M., Chopra I. Mutations affecting penicillin-binding proteins 2a, 2b and 3 in Bacillus subtilis alter cell shape and peptidoglycan metabolism. J Gen Microbiol. 1987 Jul;133(7):1733–1742. doi: 10.1099/00221287-133-7-1733. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tsuchido T., Ahn Y. H., Takano M. Lysis of Bacillus subtilis Cells by Glycerol and Sucrose Esters of Fatty Acids. Appl Environ Microbiol. 1987 Mar;53(3):505–508. doi: 10.1128/aem.53.3.505-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchido T., Hiraoka T., Takano M., Shibasaki I. Involvement of autolysin in cellular lysis of Bacillus subtilis induced by short- and medium-chain fatty acids. J Bacteriol. 1985 Apr;162(1):42–46. doi: 10.1128/jb.162.1.42-46.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ved H. S., Gustow E., Pieringer R. A. The involvement of the proteinase of Streptococcus faecium ATCC 9790 in the stimulation of its autolysin activity by dodecylglycerol. J Biol Chem. 1984 Jul 10;259(13):8122–8124. [PubMed] [Google Scholar]
- Vitković L., Cheung H. Y., Freese E. Absence of correlation between rates of cell wall turnover and autolysis shown by Bacillus subtilis mutants. J Bacteriol. 1984 Jan;157(1):318–320. doi: 10.1128/jb.157.1.318-320.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]