Abstract
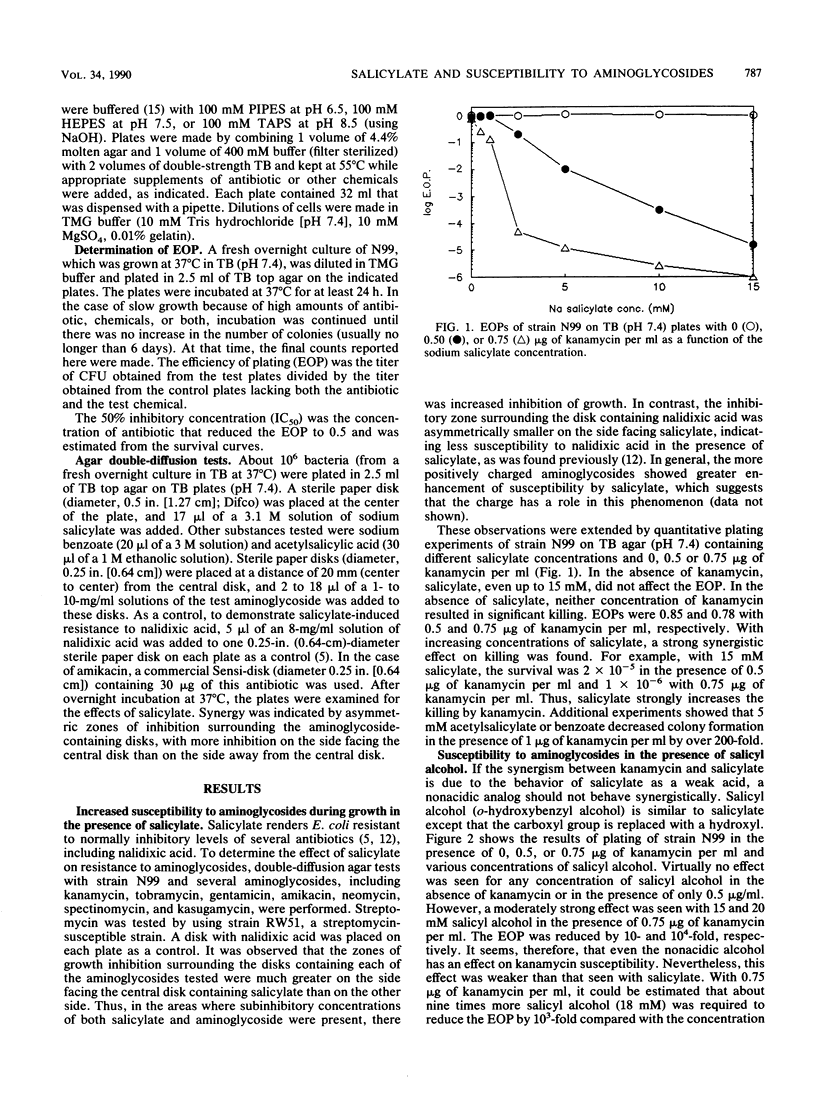
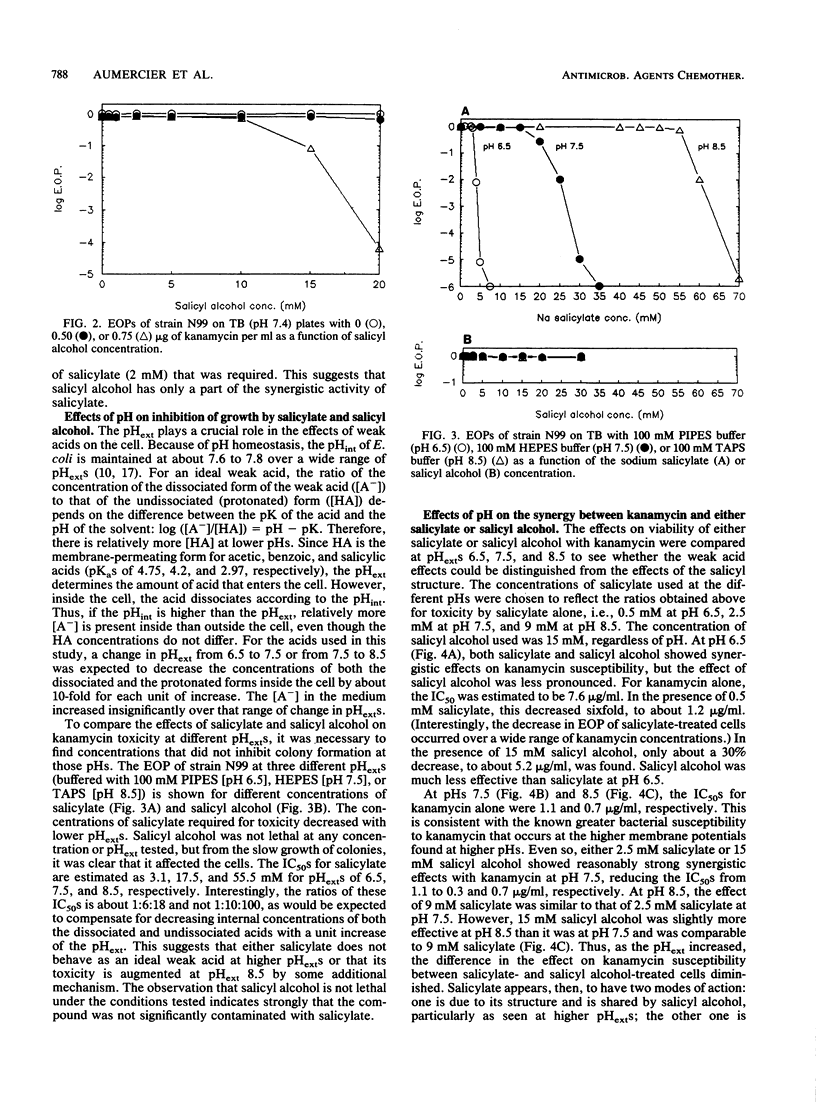
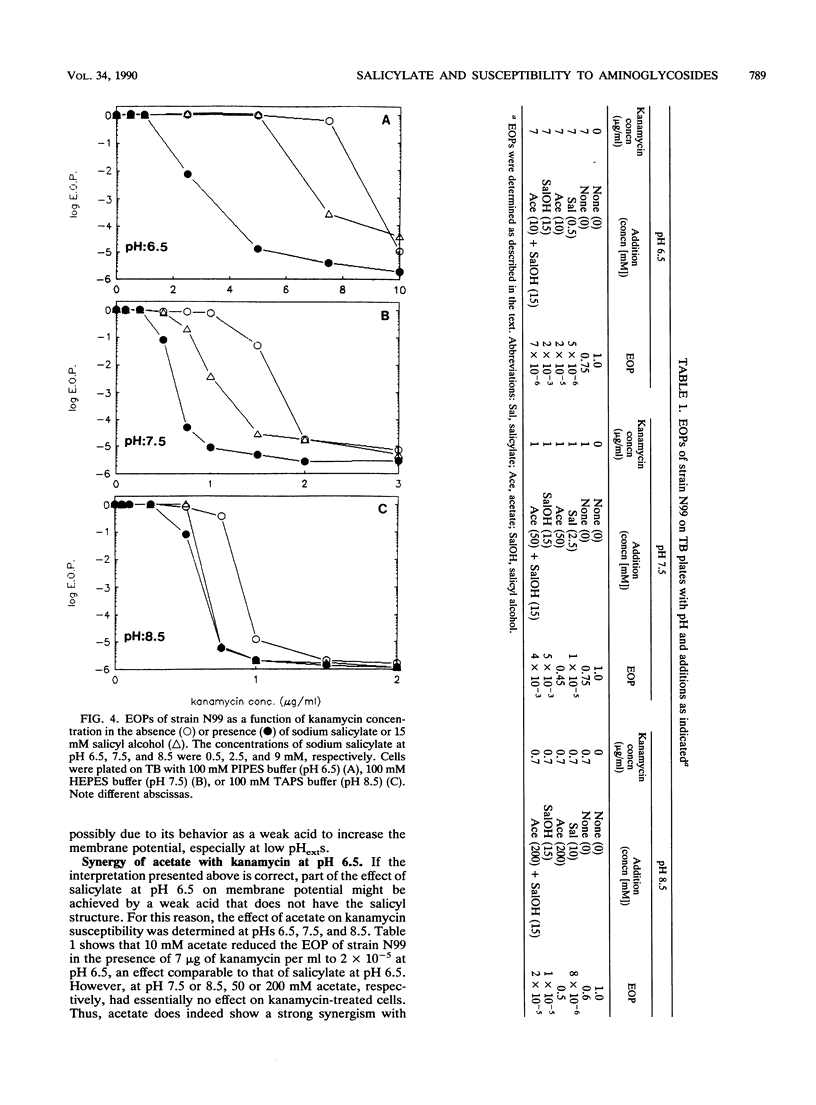
Susceptibility of Escherichia coli to kanamycin and seven other aminoglycosides has been found to be strongly potentiated by salicylate. At pH 7.5, in the presence of 15 mM salicylate and 0.5 micrograms of kanamycin per ml, the efficiency of plating of the bacteria was 2 x 10(-5), whereas there was no significant killing in the presence of kanamycin or salicylate alone. With 0.75 micrograms of kanamycin per ml, the addition of 2.5 mM salicylate was sufficient to reduce the efficiency of plating by more than 10(4)-fold. Synergistic effects were found also at pHs 6.5 and 8.5. To determine whether the action of salicylate resulted from its behavior as a weak acid or its salicyl structure, similar experiments were carried out with acetate and salicyl alcohol. Acetate, a membrane-permeating weak acid, showed a synergistic effect on kanamycin susceptibility at pH 6.5 that was comparable to the effect seen with salicylate at pH 6.5. However, acetate had no synergistic effect with kanamycin at pH 7.5 or 8.5. This is consistent with the ability of acetate to increase the membrane potential of cells and the dependence of susceptibility to kanamycin and other aminoglycosides on the membrane potential. Salicyl alcohol, which has a hydroxyl group in the place of the carboxyl group that is present in salicylate, was an effective synergist with kanamycin. It was equally effective at pHs 6.5 and 7.5 and somewhat more effective at pH 8.5. These results support the hypothesis that two effects are involved in the synergy between aminoglycosides and salicylate: a weak acid effect, possibly to increase the membrane potential, and an uncharacterized effect related to the salicyl structure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damper P. D., Epstein W. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob Agents Chemother. 1981 Dec;20(6):803–808. doi: 10.1128/aac.20.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. D., Iannetta A. Influence of serum and calcium on the bactericidal activity of gentamicin and carbenicillin on Pseudomonas aeruginosa. Appl Microbiol. 1972 Apr;23(4):775–779. doi: 10.1128/am.23.4.775-779.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Murray D. M., Chai T., Rosner J. L. Decreased permeation of cephalosporins through the outer membrane of Escherichia coli grown in salicylates. Antimicrob Agents Chemother. 1989 Apr;33(4):412–417. doi: 10.1128/aac.33.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action--with special reference to streptomycin and gentamicin. I. Antagonists and mutants. J Antimicrob Chemother. 1981 Oct;8(4):249–276. doi: 10.1093/jac/8.4.249. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. Stoichiometry of the H+-ATPase of growing and resting, aerobic Escherichia coli. Biochemistry. 1982 Oct 26;21(22):5534–5538. doi: 10.1021/bi00265a024. [DOI] [PubMed] [Google Scholar]
- Muir M. E., Wallace B. J. Isolation of mutants of Escherichia coli uncoupled in oxidative phosphorylation using hypersensitivity to streptomycin. Biochim Biophys Acta. 1979 Aug 14;547(2):218–229. doi: 10.1016/0005-2728(79)90005-7. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Nonheritable resistance to chloramphenicol and other antibiotics induced by salicylates and other chemotactic repellents in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8771–8774. doi: 10.1073/pnas.82.24.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty O. H., Hendler R. W., Shrager R. I. Simultaneous measurements of proton motive force, delta pH, membrane potential, and H+/O ratios in intact Escherichia coli. Biophys J. 1983 Sep;43(3):371–381. doi: 10.1016/S0006-3495(83)84360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Gonzalez T. N., Bartholomew F. M., Holt N. J. Mu d-directed lacZ fusions regulated by low pH in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3001–3006. doi: 10.1128/jb.169.7.3001-3006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Stock J. B., Koshland D. E., Jr Role of membrane potential and calcium in chemotactic sensing by bacteria. J Mol Biol. 1981 Jun 25;149(2):241–257. doi: 10.1016/0022-2836(81)90300-4. [DOI] [PubMed] [Google Scholar]
- Taber H. W., Mueller J. P., Miller P. F., Arrow A. S. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987 Dec;51(4):439–457. doi: 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


