Abstract
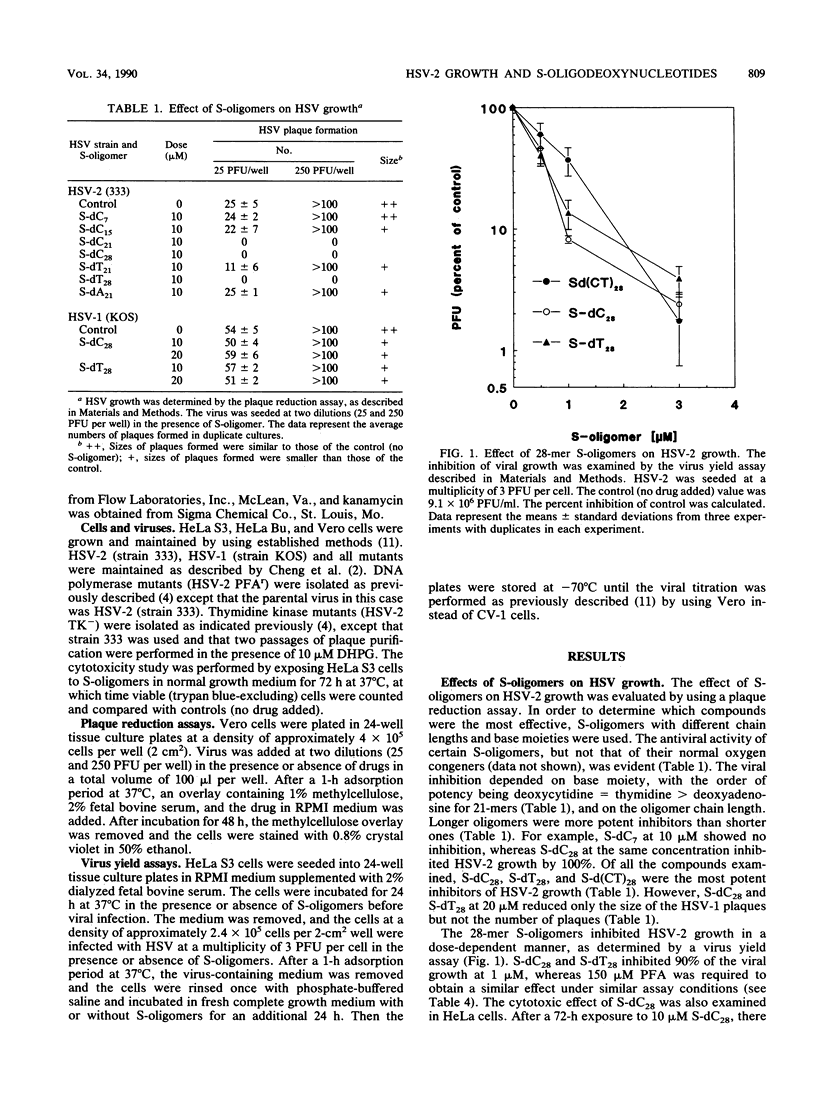
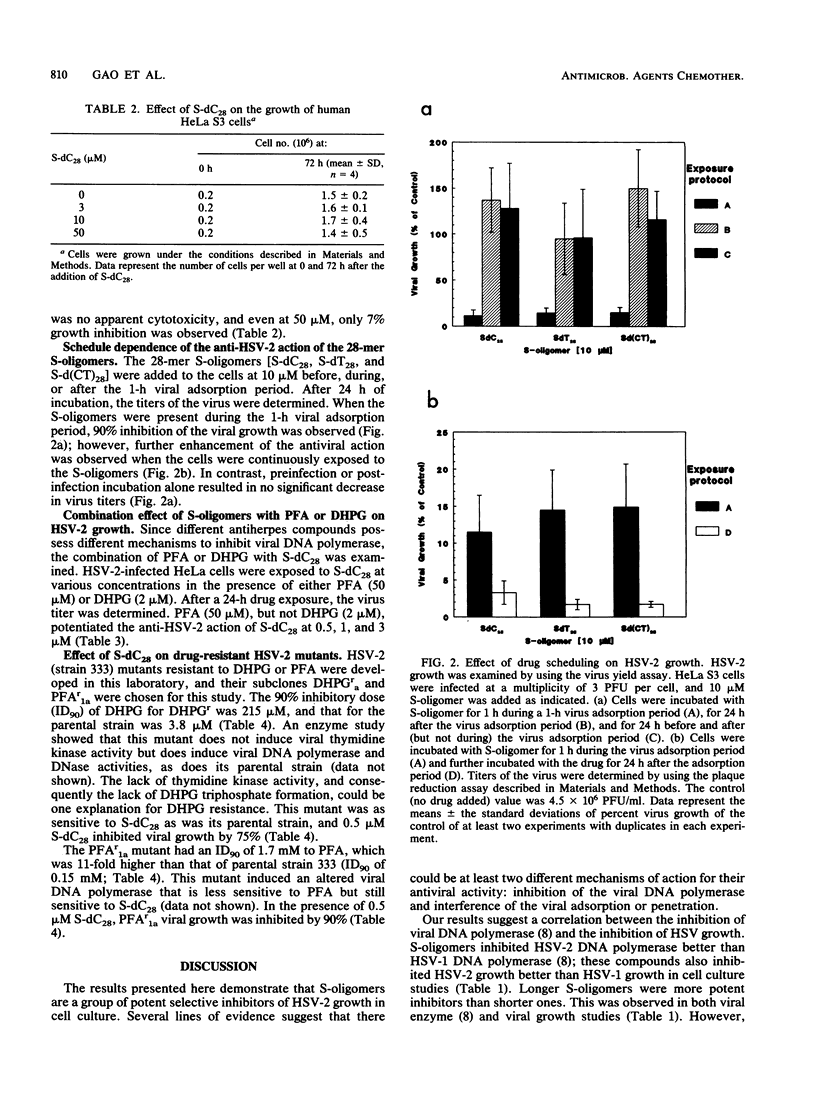
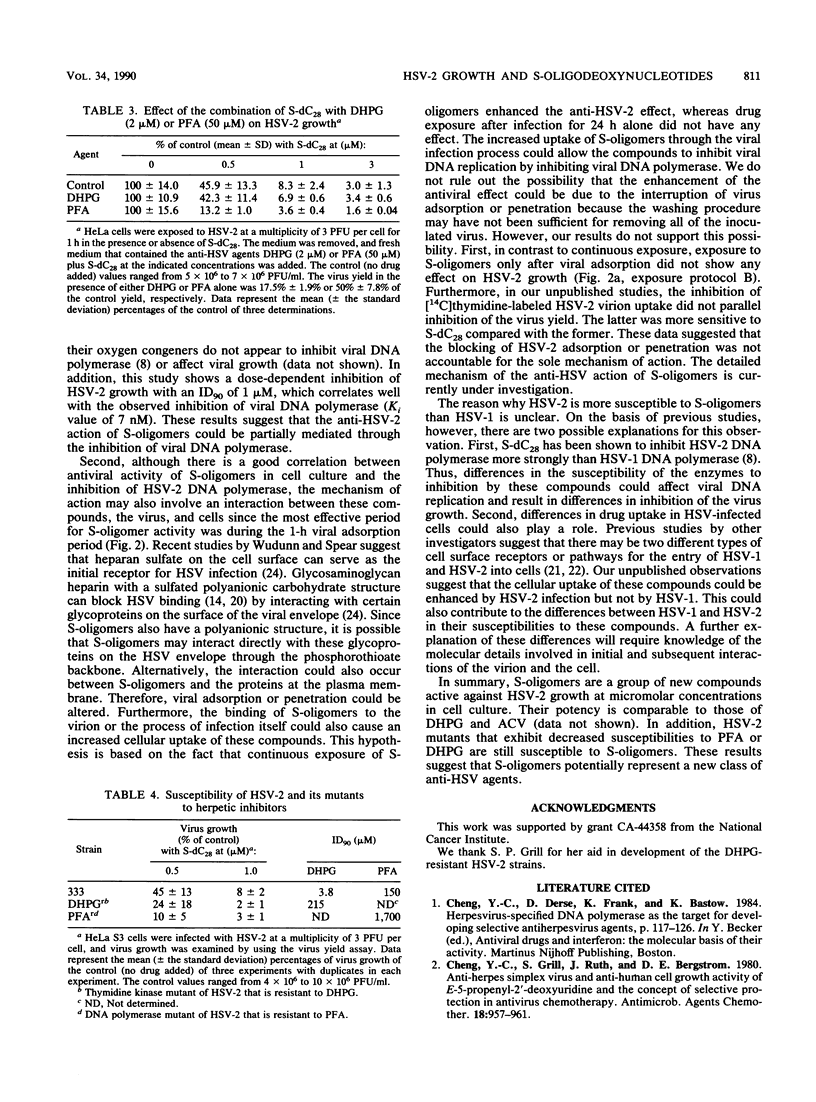
Phosphorothioate homo-oligodeoxynucleotides were found to be potent inhibitors of herpes simplex virus type 2 (HSV-2) but less potent for HSV-1 in cell culture studies. Oligomers with longer chain lengths were more active against HSV-2 than those with shorter ones. Of all the compounds examined, the 28-mer phosphorothioate homo-oligodeoxynucleotides were the strongest inhibitors of HSV-2. The degree of inhibition was related to the base moiety on the order of deoxycytidine = thymidine greater than deoxyadenosine. The inhibition of HSV-2 growth by S-dC28 was dose dependent with a 90% inhibitory dose of 1 microM. At 50 microM, S-dC28 inhibited HeLa S3 cell growth by less than 10%. The anti-HSV-2 activity was time and schedule dependent. The oligomer was most inhibitory to viral growth when present during the 1-h viral adsorption period, and this effect could be enhanced by continuous drug exposure after the adsorption period. S-dC28 was also an effective inhibitor of two HSV-2 drug-resistant mutants: a phosphonoformate-resistant mutant that induces an altered DNA polymerase and a 9-(1,3-dihydroxy-2-propoxymethyl)guanine-resistant mutant that does not induce the viral thymidine kinase. In drug combination studies, phosphonoformate was shown to potentiate the action of S-dC28 against HSV-2 growth. In conclusion, because of their potency and selectivity, phosphorothioate homo-oligodeoxynucleotides are a promising new class of anti-HSV agents.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng Y. C., Grill S., Ruth J., Bergstrom D. E. Anti-herpes simplex virus and anti-human cell growth activity of E-5-propenyl-2'-deoxyuridine and the concept of selective protection in antivirus chemotherapy. Antimicrob Agents Chemother. 1980 Dec;18(6):957–961. doi: 10.1128/aac.18.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Bastow K. F., Cheng Y. Characterization of the DNA polymerases induced by a group of herpes simplex virus type I variants selected for growth in the presence of phosphonoformic acid. J Biol Chem. 1982 Sep 10;257(17):10251–10260. [PubMed] [Google Scholar]
- Derse D., Cheng Y. C., Furman P. A., St Clair M. H., Elion G. B. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J Biol Chem. 1981 Nov 25;256(22):11447–11451. [PubMed] [Google Scholar]
- Eriksson B., Larsson A., Helgstrand E., Johansson N. G., Oberg B. Pyrophosphate analogues as inhibitors of herpes simplex virus type 1 DNA polymerase. Biochim Biophys Acta. 1980 Mar 28;607(1):53–64. doi: 10.1016/0005-2787(80)90220-8. [DOI] [PubMed] [Google Scholar]
- Frank K. B., Chiou J. F., Cheng Y. C. Interaction of herpes simplex virus-induced DNA polymerase with 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1984 Feb 10;259(3):1566–1569. [PubMed] [Google Scholar]
- Frank K. B., Derse D. D., Bastow K. F., Cheng Y. C. Novel interaction of aphidicolin with herpes simplex virus DNA polymerase and polymerase-associated exonuclease. J Biol Chem. 1984 Nov 10;259(21):13282–13286. [PubMed] [Google Scholar]
- Gao W. Y., Stein C. A., Cohen J. S., Dutschman G. E., Cheng Y. C. Effect of phosphorothioate homo-oligodeoxynucleotides on herpes simplex virus type 2-induced DNA polymerase. J Biol Chem. 1989 Jul 5;264(19):11521–11526. [PubMed] [Google Scholar]
- Germershausen J., Bostedor R., Field A. K., Perry H., Liou R., Bull H., Tolman R. L., Karkas J. D. A comparison of the antiviral agents 2'-nor-2'-deoxyguanosine and acyclovir: uptake and phosphorylation in tissue culture and kinetics of in vitro inhibition of viral and cellular DNA polymerases by their respective triphosphates. Biochem Biophys Res Commun. 1983 Oct 31;116(2):360–367. doi: 10.1016/0006-291x(83)90530-2. [DOI] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Lewandowski G. A., Grill S. P., Fisher M. H., Dutschman G. E., Efange S. M., Bardos T. J., Cheng Y. C. Anti-herpes simplex virus activity of 5-substituted 2-pyrimidinone nucleosides. Antimicrob Agents Chemother. 1989 Mar;33(3):340–344. doi: 10.1128/aac.33.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar C., Stein C. A., Cohen J. S., Broder S., Wilson S. H. Stepwise mechanism of HIV reverse transcriptase: primer function of phosphorothioate oligodeoxynucleotide. Biochemistry. 1989 Feb 7;28(3):1340–1346. doi: 10.1021/bi00429a060. [DOI] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Kibrick S. Inhibitory effect of heparin on herpes simplex virus. J Bacteriol. 1964 May;87(5):1060–1066. doi: 10.1128/jb.87.5.1060-1066.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Mechanism of inhibition of herpes simplex virus and vaccinia virus DNA polymerases by aphidicolin, a highly specific inhibitor of DNA replication in eucaryotes. J Virol. 1980 Nov;36(2):457–464. doi: 10.1128/jvi.36.2.457-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno J. M., Lee L. F., Boezi J. A. Inhibition of herpesvirus replication and herpesvirus-induced deoxyribonucleic acid polymerase by phosphonoformate. Antimicrob Agents Chemother. 1978 Feb;13(2):188–192. doi: 10.1128/aac.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INHIBITION OF HERPES VIRUS BY NATURAL AND SYNTHETIC ACID POLYSACCHARIDES. Proc Soc Exp Biol Med. 1964 May;116:140–144. doi: 10.3181/00379727-116-29183. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Svennerholm B., Lycke E. Evidence for herpes simplex virus type-selective receptors on cellular plasma membranes. J Gen Virol. 1979 Jul;44(1):217–225. doi: 10.1099/0022-1317-44-1-217. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Svennerholm B., Sandberg M., Hamberger A., Lycke E. Differences in attachment between herpes simplex type 1 and type 2 viruses to neurons and glial cells. Infect Immun. 1980 Jun;28(3):675–680. doi: 10.1128/iai.28.3.675-680.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



