Abstract
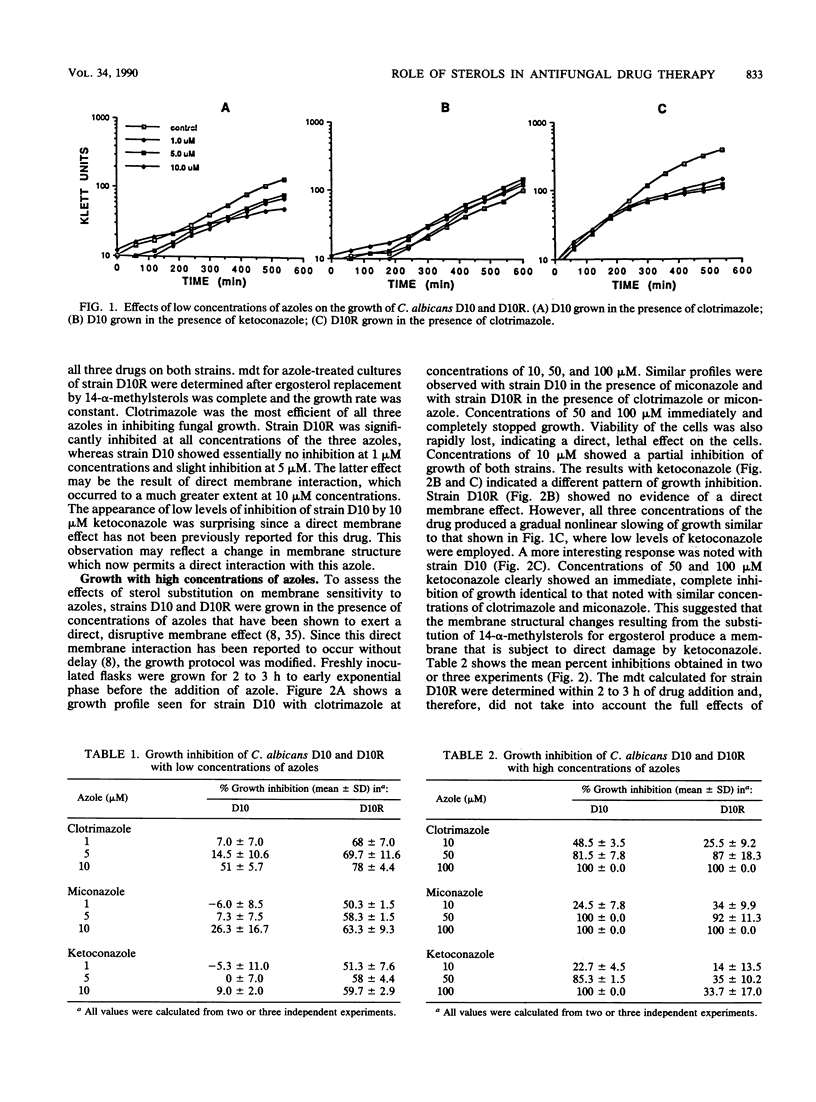
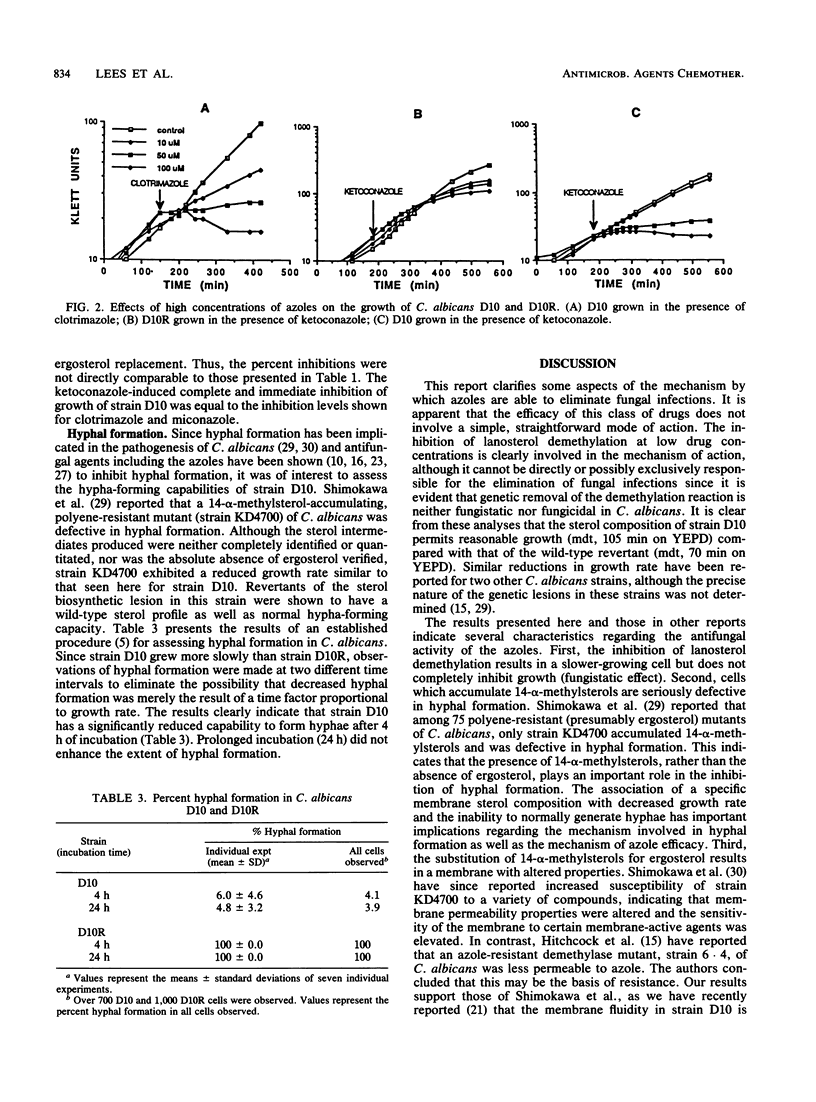
A cytochrome P-450-deficient mutant of Candida albicans, strain D10, was employed to study the mode of action of imidazole antifungal agents. This mutant accumulates exclusively 14-alpha-methylsterols, resulting in a sterol profile which mimics that of azole-treated wild-type strains. Since the widely accepted primary effect of imidazoles is the inhibition of cytochrome P-450-mediated demethylation of the ergosterol precursor lanosterol, strain D10 and its wild-type revertant, strain D10R, were grown in the presence of concentrations of clotrimazole, miconazole, and ketoconazole known to inhibit demethylation. The growth of strain D10 was unaffected by these antifungal agents, while that of strain D10R was significantly reduced. At higher azole concentrations (which are known to exert a direct, disruptive action on the cell membrane), the growth of both strains was immediately and completely inhibited by clotrimazole and miconazole. Ketoconazole was membrane disruptive only for strain D10; this is the first report of a direct membrane effect for this drug. Because hyphal formation has been implicated in the pathogenesis of C. albicans and because it has been shown to be inhibited by azoles, the hypha-forming capability of strain D10 was examined. Strain D10 was shown to be seriously defective in hyphal formation, suggesting that this function may be dependent on the 14-alpha-demethylation of lanosterol. The results of this study suggest that inhibition of lanosterol demethylation per se is neither fungicidal nor fungistatic, although the growth rate is reduced. In addition, the substitution of 14-alpha-methylsterols for ergosterol results in defective hyphal formation and in a cell that is more susceptible to membrane-active agents such as ketoconazole.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bard M., Lees N. D., Barbuch R. J., Sanglard D. Characterization of a cytochrome P450 deficient mutant of Candida albicans. Biochem Biophys Res Commun. 1987 Sep 15;147(2):794–800. doi: 10.1016/0006-291x(87)91000-x. [DOI] [PubMed] [Google Scholar]
- Bard M., Lees N. D., Burrows L. S., Kleinhans F. W. Differences in crystal violet uptake and cation-induced death among yeast sterol mutants. J Bacteriol. 1978 Sep;135(3):1146–1148. doi: 10.1128/jb.135.3.1146-1148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs W. H. Regulation of the direct fungicidal action of miconazole against Candida albicans ATCC 26790. Mycoses. 1988 Apr;31(4):226–228. [PubMed] [Google Scholar]
- Beggs W. H. Requirement for nonprotonated drug molecules in the direct lethal action of miconazole against Candida albicans. Mycopathologia. 1988 Aug;103(2):91–94. doi: 10.1007/BF00441264. [DOI] [PubMed] [Google Scholar]
- Borgers M., De Brabander M., Van Den Bossche H., Van Cutsem J. Promotion of pseudomycelium formation of Candida albicans in culture: a morphological study of the effects of miconazole and ketoconazole. Postgrad Med J. 1979 Sep;55(647):687–691. doi: 10.1136/pgmj.55.647.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobon G. S., Haslam J. M. The effect of altered membrane sterol composition on the temperature dependence of yeast mitochondrial ATPase. Biochem Biophys Res Commun. 1973 May 1;52(1):320–326. doi: 10.1016/0006-291x(73)90990-x. [DOI] [PubMed] [Google Scholar]
- Cope J. E. Mode of action of miconazole on Candida albicans: effect on growth, viability and K+ release. J Gen Microbiol. 1980 Jul;119(1):245–251. doi: 10.1099/00221287-119-1-245. [DOI] [PubMed] [Google Scholar]
- Dahl C., Biemann H. P., Dahl J. A protein kinase antigenically related to pp60v-src possibly involved in yeast cell cycle control: positive in vivo regulation by sterol. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4012–4016. doi: 10.1073/pnas.84.12.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Dix B. A., Smith S. A., Freudenberger J., Funke P. T. Effect of antifungal agents on lipid biosynthesis and membrane integrity in Candida albicans. Antimicrob Agents Chemother. 1987 Jan;31(1):46–51. doi: 10.1128/aac.31.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. J., Sisler H. D. Effects of miconazole and dodecylimidazole on sterol biosynthesis in Ustilago maydis. Antimicrob Agents Chemother. 1979 Apr;15(4):603–607. doi: 10.1128/aac.15.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock C. A., Barrett-Bee K. J., Russell N. J. The lipid composition and permeability to azole of an azole- and polyene-resistant mutant of Candida albicans. J Med Vet Mycol. 1987 Feb;25(1):29–37. doi: 10.1080/02681218780000041. [DOI] [PubMed] [Google Scholar]
- Hitchcock C. A., Barrett-Bee K. J., Russell N. J. The lipid composition and permeability to the triazole antifungal antibiotic ICI 153066 of serum-grown mycelial cultures of Candida albicans. J Gen Microbiol. 1989 Jul;135(7):1949–1955. doi: 10.1099/00221287-135-7-1949. [DOI] [PubMed] [Google Scholar]
- Kleinhans F. W., Lees N. D., Bard M., Haak R. A., Woods R. A. ESR determinations of membrane permeability in a yeast sterol mutant. Chem Phys Lipids. 1979 Jan-Feb;23(2):143–154. doi: 10.1016/0009-3084(79)90042-2. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Kirsch D. R., Kelly R. The molecular genetics of Candida albicans. Microbiol Sci. 1988 Feb;5(2):58–63. [PubMed] [Google Scholar]
- Lees N. D., Bard M., Kemple M. D., Haak R. A., Kleinhans F. W. ESR determination of membrane order parameter in yeast sterol mutants. Biochim Biophys Acta. 1979 Jun 2;553(3):469–475. doi: 10.1016/0005-2736(79)90302-x. [DOI] [PubMed] [Google Scholar]
- Lees N. D., Kleinhans F. W., Broughton M. C., Pennington D. E., Ricker V. A., Bard M. Membrane fluidity alterations in a cytochrome P-450-deficient mutant of Candida albicans. Steroids. 1989 Mar-May;53(3-5):567–578. doi: 10.1016/0039-128x(89)90032-9. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Cockayne A., Hayward J., Abbott A. B. Effects of imidazole- and triazole-derivative antifungal compounds on the growth and morphological development of Candida albicans hyphae. J Gen Microbiol. 1985 Oct;131(10):2581–2589. doi: 10.1099/00221287-131-10-2581. [DOI] [PubMed] [Google Scholar]
- Pesti M., Paku S., Novák E. K. Some characteristics of nystatin-resistant sterol mutants of Candida albicans. Acta Microbiol Acad Sci Hung. 1982;29(1):55–66. [PubMed] [Google Scholar]
- Pierce A. M., Pierce H. D., Jr, Unrau A. M., Oehlschlager A. C. Lipid composition and polyene antibiotic resistance of Candida albicans mutants. Can J Biochem. 1978 Feb;56(2):135–142. doi: 10.1139/o78-023. [DOI] [PubMed] [Google Scholar]
- Saag M. S., Dismukes W. E. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother. 1988 Jan;32(1):1–8. doi: 10.1128/aac.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaude M., Ackerbauer H., Mieth H. Inhibitory effect of antifungal agents on germ tube formation in Candida albicans. Mykosen. 1987 Jun;30(6):281–287. doi: 10.1111/j.1439-0507.1987.tb03980.x. [DOI] [PubMed] [Google Scholar]
- Shigematsu M. L., Uno J., Arai T. Effect of ketoconazole on isolated mitochondria from Candida albicans. Antimicrob Agents Chemother. 1982 Jun;21(6):919–924. doi: 10.1128/aac.21.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa O., Kato Y., Nakayama H. Accumulation of 14-methyl sterols and defective hyphal growth in Candida albicans. J Med Vet Mycol. 1986 Aug;24(4):327–336. doi: 10.1080/02681218680000481. [DOI] [PubMed] [Google Scholar]
- Shimokawa O., Kato Y., Nakayama H. Increased drug sensitivity in Candida albicans cells accumulating 14-methylated sterols. J Med Vet Mycol. 1986 Dec;24(6):481–483. [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Heterogeneity of action of mechanisms among antimycotic imidazoles. Antimicrob Agents Chemother. 1981 Jul;20(1):71–74. doi: 10.1128/aac.20.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surarit R., Shepherd M. G. The effects of azole and polyene antifungals on the plasma membrane enzymes of Candida albicans. J Med Vet Mycol. 1987 Dec;25(6):403–413. doi: 10.1080/02681218780000491. [DOI] [PubMed] [Google Scholar]
- Thompson E. D., Parks L. W. Effect of altered sterol composition on growth characteristics of Saccharomyces cerevisiae. J Bacteriol. 1974 Nov;120(2):779–784. doi: 10.1128/jb.120.2.779-784.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche H., Ruysschaert J. M., Defrise-Quertain F., Willemsens G., Cornelissen F., Marichal P., Cools W., Van Cutsem J. The interaction of miconazole and ketoconazole with lipids. Biochem Pharmacol. 1982 Aug 15;31(16):2609–2617. doi: 10.1016/0006-2952(82)90707-9. [DOI] [PubMed] [Google Scholar]
- Van den Bossche H., Willemsens G., Cools W., Cornelissen F., Lauwers W. F., van Cutsem J. M. In vitro and in vivo effects of the antimycotic drug ketoconazole on sterol synthesis. Antimicrob Agents Chemother. 1980 Jun;17(6):922–928. doi: 10.1128/aac.17.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Bossche H., Willemsens G., Marichal P. Anti-Candida drugs--the biochemical basis for their activity. Crit Rev Microbiol. 1987;15(1):57–72. doi: 10.3109/10408418709104448. [DOI] [PubMed] [Google Scholar]


