Abstract
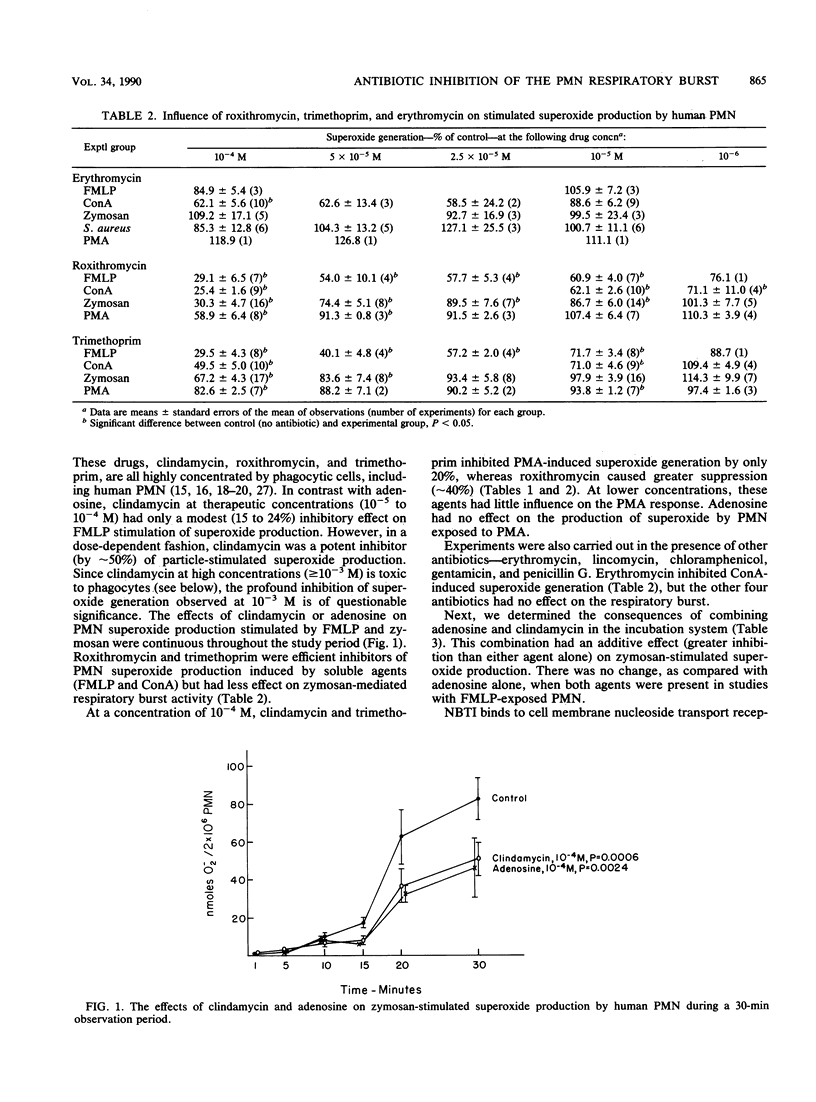
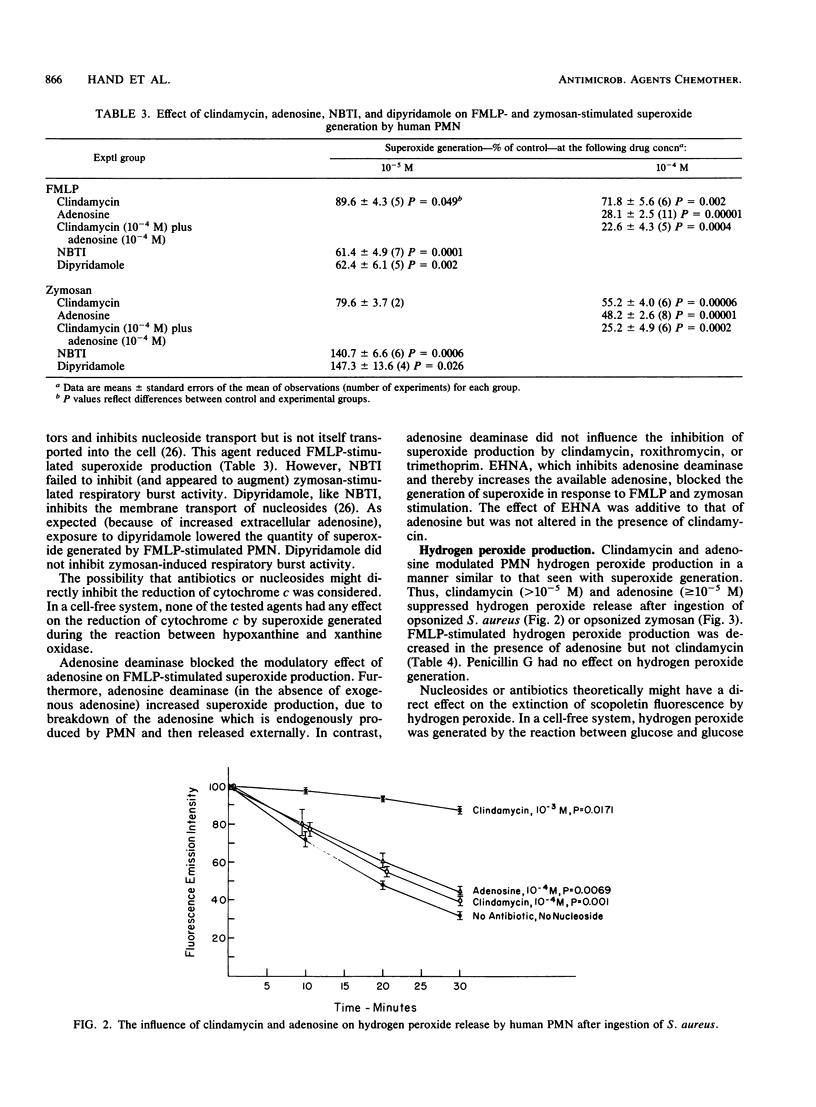
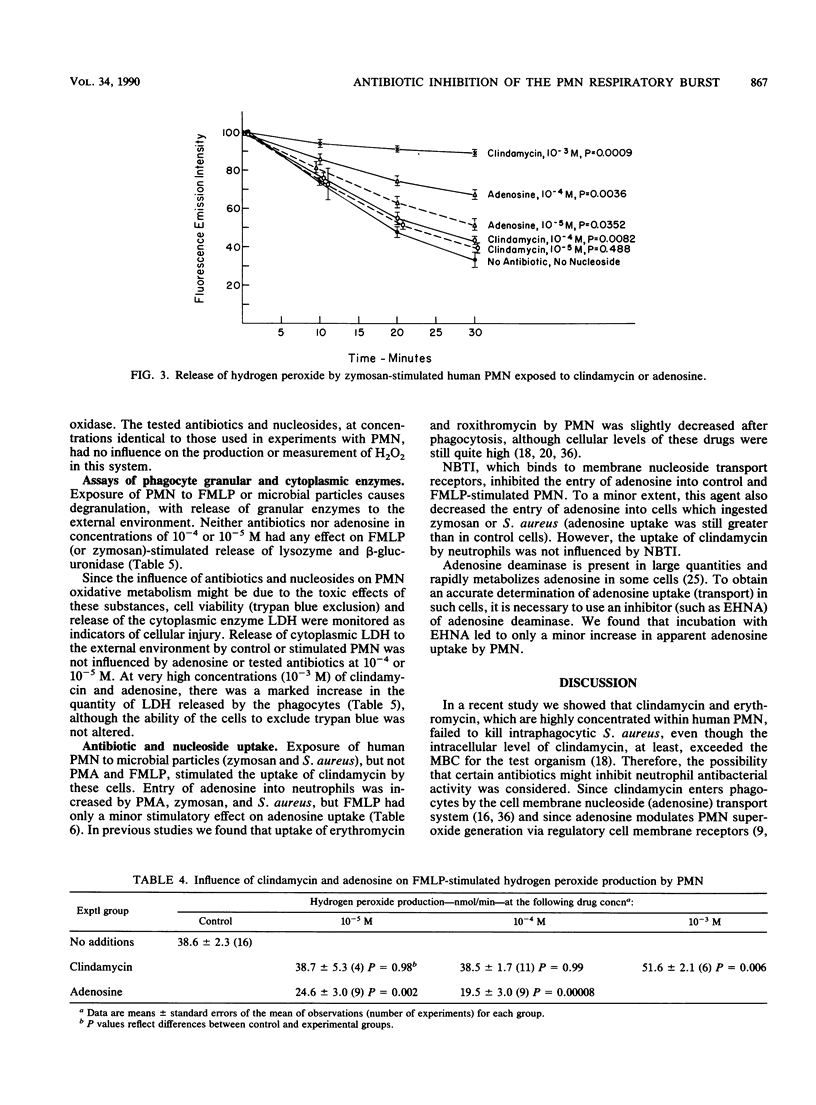
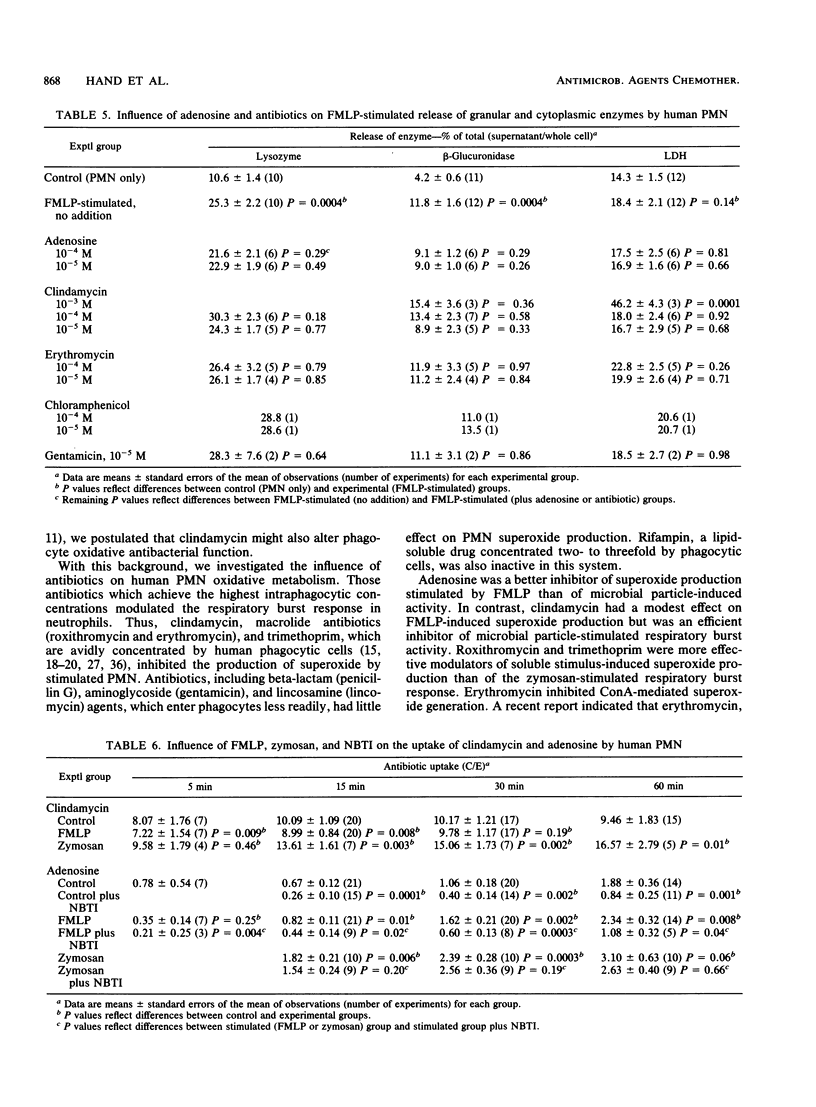
Recently we found that certain antibiotics which are markedly concentrated by human polymorphonuclear leukocytes (PMN) failed to kill susceptible, intraphagocytic Staphylococcus aureus, even though cellular drug levels were quite high. The possibility that specific antibiotics might adversely affect phagocyte antibacterial function was considered. Thus, we studied the effects of multiple antibiotics and adenosine, a known modulator of the PMN respiratory burst response, on neutrophil antibacterial function. At nontoxic concentrations, these drugs had no effect on degranulation in stimulated PMN. Adenosine was a potent inhibitor of formyl-methionyl-leucyl-phenylalanine (FMPL)-stimulated superoxide and hydrogen peroxide generation in PMN but produced less inhibition of microbial particle-induced respiratory burst activity. Three of the tested antibiotics, all of which reach high concentrations in phagocytic cells, had a marked modulatory effect on the PMN respiratory burst. Clindamycin, which enters phagocytes by the cell membrane adenosine (nucleoside) transport system, had only a modest effect on FMLP-mediated superoxide production but inhibited the microbial particle-induced response by approximately 50%. Roxithromycin and trimethoprim were efficient inhibitors of PMN superoxide generation stimulated by FMLP and concanavalin A (also inhibited by erythromycin) but had less effect on zymosan-mediated respiratory burst activity. Antibiotics which entered phagocytes less readily had no effect on the respiratory burst response in PMN. These results, as well as those of experiments with inhibitors of cell membrane nucleoside receptors, indicated that the antibiotic effect is mediated through intraphagocytic pathways. The possibility that antibiotic-associated inhibition of the PMN respiratory burst response might alter leukocyte antimicrobial and inflammatory function deserves further evaluation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMADOR E., DORFMAN L. E., WACKER W. E. SERUM LACTIC DEHYDROGENASE ACTIVITY: AN ANALYTICAL ASSESSMENT OF CURRENT ASSAYS. Clin Chem. 1963 Aug;12:391–399. [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst oxidase. Hematol Oncol Clin North Am. 1988 Jun;2(2):201–212. [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Crawford D. R., Schneider D. L. Identification of ubiquinone-50 in human neutrophils and its role in microbicidal events. J Biol Chem. 1982 Jun 25;257(12):6662–6668. [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kubersky S. M., Weissmann G., Hirschhorn R. Engagement of adenosine receptors inhibits hydrogen peroxide (H2O2-) release by activated human neutrophils. Clin Immunol Immunopathol. 1987 Jan;42(1):76–85. doi: 10.1016/0090-1229(87)90174-7. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Rosenstein E. D., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985 Aug;135(2):1366–1371. [PubMed] [Google Scholar]
- Daly J. W., Bruns R. F., Snyder S. H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981 May 11;28(19):2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Lefker B. A. Deficient flavoprotein component of the NADPH-dependent O2-.-generating oxidase in the neutrophils from three male patients with chronic granulomatous disease. J Clin Invest. 1984 Mar;73(3):701–705. doi: 10.1172/JCI111262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., Corwin R. W., Steinberg T. H., Grossman G. D. Uptake of antibiotics by human alveolar macrophages. Am Rev Respir Dis. 1984 Jun;129(6):933–937. doi: 10.1164/arrd.1984.129.6.933. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Contrasts between phagocyte antibiotic uptake and subsequent intracellular bactericidal activity. Antimicrob Agents Chemother. 1986 Jan;29(1):135–140. doi: 10.1128/aac.29.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Effect of erythrocyte ingestion on macrophage antibacterial function. Infect Immun. 1983 Jun;40(3):917–923. doi: 10.1128/iai.40.3.917-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Membrane transport of clindamycin in alveolar macrophages. Antimicrob Agents Chemother. 1982 Feb;21(2):241–247. doi: 10.1128/aac.21.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. The entry of antibiotics into human monocytes. J Antimicrob Chemother. 1989 May;23(5):681–689. doi: 10.1093/jac/23.5.681. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N., Holman J. W. Entry of roxithromycin (RU 965), imipenem, cefotaxime, trimethoprim, and metronidazole into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Oct;31(10):1553–1557. doi: 10.1128/aac.31.10.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner M. S., Styrt B. Alkalinization of the intralysosomal pH by clindamycin and its effects on neutrophil function. J Antimicrob Chemother. 1983 Oct;12 (Suppl 100):39–50. doi: 10.1093/jac/12.suppl_c.39. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Styrt B. Alkalinizing the intralysosomal pH inhibits degranulation of human neutrophils. J Clin Invest. 1983 Nov;72(5):1793–1800. doi: 10.1172/JCI111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R. E., Jr, Dawicki D. D., Agarwal K. C., Chen S. F., Stoeckler J. D. Role of nucleoside transport in drug action. The adenosine deaminase inhibitor, deoxycoformycin, and the antiplatelet drugs, dipyridamole and dilazep. Ann N Y Acad Sci. 1985;451:188–203. doi: 10.1111/j.1749-6632.1985.tb27110.x. [DOI] [PubMed] [Google Scholar]
- Prokesch R. C., Hand W. L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982 Mar;21(3):373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Cross A. R., Garcia R. C., Borregaard N., Valerius N. H., Soothill J. F., Jones O. T. Absence of cytochrome b-245 in chronic granulomatous disease. A multicenter European evaluation of its incidence and relevance. N Engl J Med. 1983 Feb 3;308(5):245–251. doi: 10.1056/NEJM198302033080503. [DOI] [PubMed] [Google Scholar]
- Segal A. W. Cytochrome b-245 and its involvement in the molecular pathology of chronic granulomatous disease. Hematol Oncol Clin North Am. 1988 Jun;2(2):213–223. [PubMed] [Google Scholar]
- Snyderman R., Verghese M. W. Leukocyte activation by chemoattractant receptors: roles of a guanine nucleotide regulatory protein and polyphosphoinositide metabolism. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S562–S569. doi: 10.1093/clinids/9.supplement_5.s562. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Gierschik P., Levine M. A., Downs R. W., Jr Clinical implications of guanine nucleotide-binding proteins as receptor-effector couplers. N Engl J Med. 1985 Jan 3;312(1):26–33. doi: 10.1056/NEJM198501033120106. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Hand W. L. Effects of phagocytosis on antibiotic and nucleoside uptake by human polymorphonuclear leukocytes. J Infect Dis. 1984 Mar;149(3):397–403. doi: 10.1093/infdis/149.3.397. [DOI] [PubMed] [Google Scholar]


