Abstract
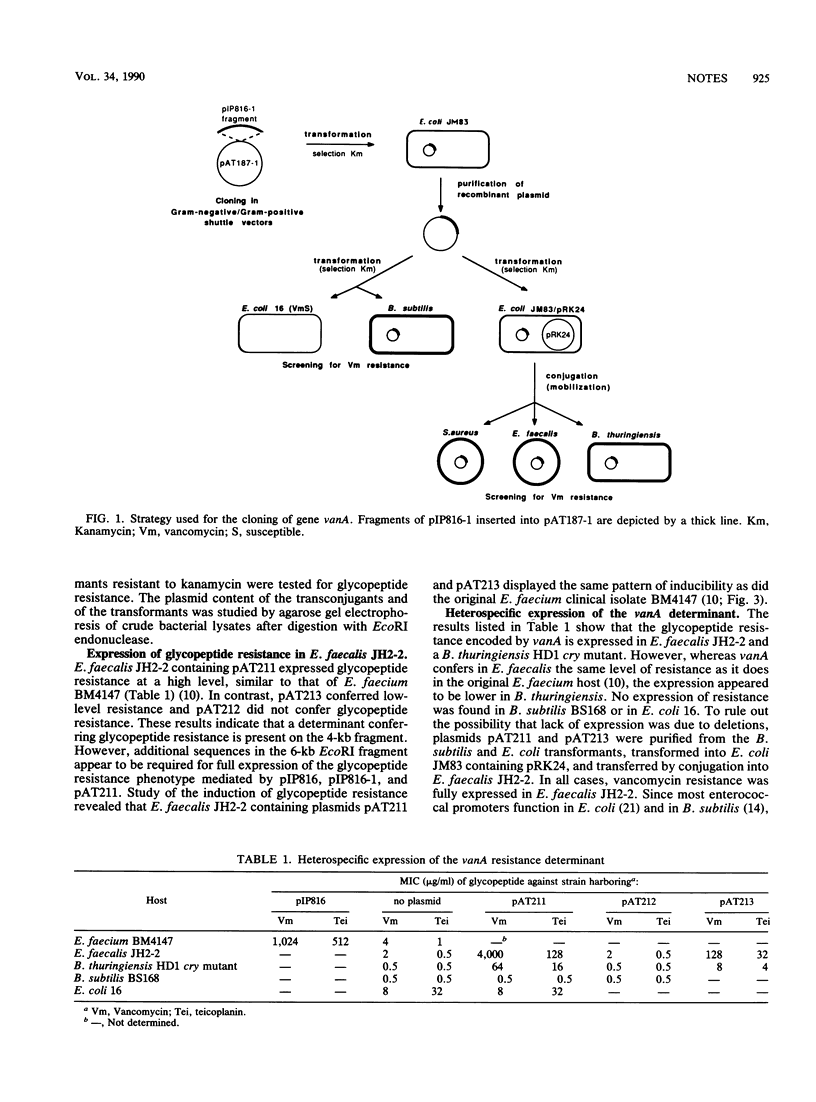
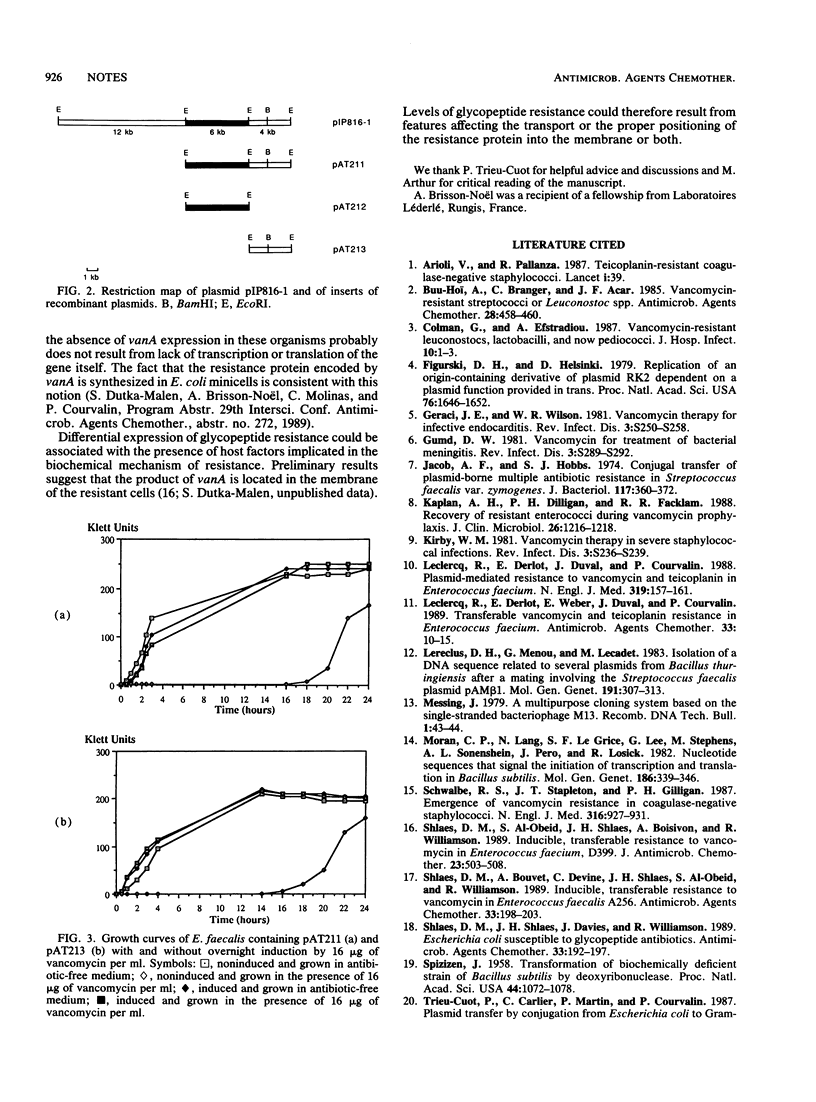
Fragments of plasmid pIP816, which confers high-level glycopeptide resistance in Enterococcus faecium BM4147, were cloned into a conjugative gram-negative-gram-positive shuttle vector. The resulting hybrids were transferred by conjugation from Escherichia coli to Enterococcus faecalis and Bacillus thuringiensis. A 4-kilobase EcoRI fragment from pIP816 was found to confer vancomycin resistance in these hosts but not in E. coli or Bacillus subtilis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arioli V., Pallanza R. Teicoplanin-resistant coagulase-negative staphylococci. Lancet. 1987 Jan 3;1(8523):39–39. doi: 10.1016/s0140-6736(87)90724-0. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï A., Branger C., Acar J. F. Vancomycin-resistant streptococci or Leuconostoc sp. Antimicrob Agents Chemother. 1985 Sep;28(3):458–460. doi: 10.1128/aac.28.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman G., Efstratiou A. Vancomycin-resistant leuconostocs, lactobacilli and now pediococci. J Hosp Infect. 1987 Jul;10(1):1–3. doi: 10.1016/0195-6701(87)90025-9. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci J. E., Wilson W. R. Vancomycin therapy for infective endocarditis. Rev Infect Dis. 1981 Nov-Dec;3 Suppl:S250–S258. [PubMed] [Google Scholar]
- Gump D. W. Vancomycin for treatment of bacterial meningitis. Rev Infect Dis. 1981 Nov-Dec;3 Suppl:S289–S292. [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby W. M. Vancomycin therapy in severe staphylococcal infections. Rev Infect Dis. 1981 Nov-Dec;3 Suppl:S236–S239. [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lereclus D., Menou G., Lecadet M. M. Isolation of a DNA sequence related to several plasmids from Bacillus thuringiensis after a mating involving the Streptococcus faecalis plasmid pAM beta 1. Mol Gen Genet. 1983;191(2):307–313. doi: 10.1007/BF00334831. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Al-Obeid S., Shlaes J. H., Boisivon A., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecium, D399. J Antimicrob Chemother. 1989 Apr;23(4):503–508. doi: 10.1093/jac/23.4.503. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Shlaes J. H., Davies J., Williamson R. Escherichia coli susceptible to glycopeptide antibiotics. Antimicrob Agents Chemother. 1989 Feb;33(2):192–197. doi: 10.1128/aac.33.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Klier A., Courvalin P. DNA sequences specifying the transcription of the streptococcal kanamycin resistance gene in Escherichia coli and Bacillus subtilis. Mol Gen Genet. 1985;198(2):348–352. doi: 10.1007/BF00383017. [DOI] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C. The antibacterial action of vancomycin. Rev Infect Dis. 1981 Nov-Dec;3 Suppl:S210–S215. [PubMed] [Google Scholar]
- Wilson A. P., O'Hare M. D., Felmingham D., Grüneberg R. N. Teicoplanin-resistant coagulase-negative staphylococcus. Lancet. 1986 Oct 25;2(8513):973–973. doi: 10.1016/s0140-6736(86)90622-7. [DOI] [PubMed] [Google Scholar]