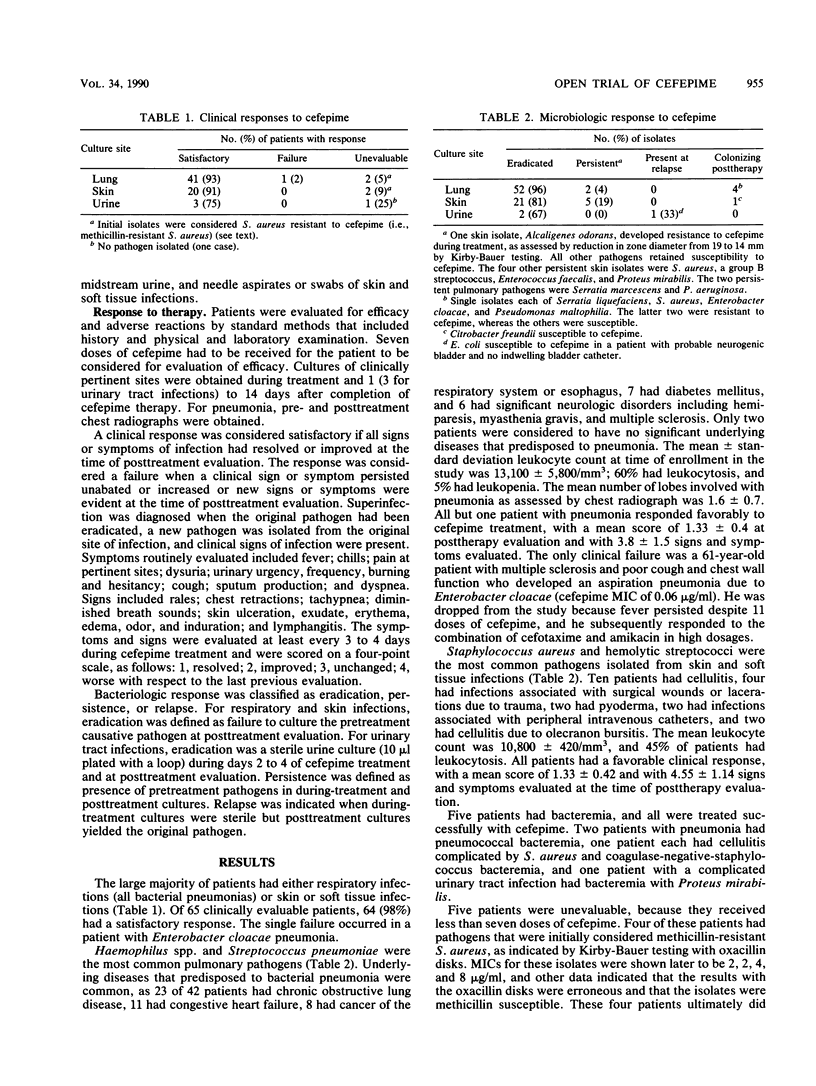
Abstract
The safety and efficacy of cefepime, a new broad-spectrum, semisynthetic parenteral cephem antibiotic, were evaluated in an open trial at a single hospital. Seventy patients were treated with cefepime: 44 had lower respiratory tract infections, 4 had urinary tract infections, and 22 had skin or soft tissue infections. Of 65 clinically evaluable patients, 64 (98%) had satisfactory responses. No mortality or superinfections occurred. Of 57 respiratory and urinary tract pathogens, 54 (95%) were eradicated and 3 (5%) persisted after therapy. Five bacteremias (two with Streptococcus pneumoniae and one each with Staphylococcus aureus, Proteus mirabilis, and a coagulase-negative staphylococcus) were eradicated. MICs ranged from 1 to 8 micrograms/ml for 13 S. aureus and 9 Pseudomonas aeruginosa isolates and were less than or equal to 0.125 micrograms/ml for 10 streptococcal isolates. Adverse effects occurred in two patients: transient diarrhea and Clostridium difficile toxin in the stool in one patient and loose bowel movements and increased transaminases in the other patient. Cefepime appeared to be well tolerated in humans and was effective against a wide range of isolates, including S. aureus and P. aeruginosa.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P., Ho D. H., LeBlanc B. In vitro studies of BMY-28142, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 Feb;27(2):265–269. doi: 10.1128/aac.27.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Tomc J., Dougherty T. J., DeOrio F. J., Simich-Jacobson V., Kessler R. E. Activity of cefepime against ceftazidime- and cefotaxime-resistant gram-negative bacteria and its relationship to beta-lactamase levels. Antimicrob Agents Chemother. 1989 Apr;33(4):498–502. doi: 10.1128/aac.33.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Bies M., Buck R. E., Chisholm D. R., Pursiano T. A., Tsai Y. H., Misiek M., Price K. E., Leitner F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 Feb;27(2):207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. J., Bihl J. A., Schell R. F., LeFrock J. L., Weber S. J. Antimicrobial activities of BMY-28142, cefbuperazone, and cefpiramide compared with those of other cephalosporins. Antimicrob Agents Chemother. 1984 Oct;26(4):585–590. doi: 10.1128/aac.26.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Bayer A. S. Efficacy of BMY-28142 in experimental bacteremia and meningitis caused by Escherichia coli and group B streptococci. Antimicrob Agents Chemother. 1985 Jul;28(1):51–54. doi: 10.1128/aac.28.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps D. J., Carlton D. D., Farrell C. A., Kessler R. E. Affinity of cephalosporins for beta-lactamases as a factor in antibacterial efficacy. Antimicrob Agents Chemother. 1986 May;29(5):845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Täuber M. G., Hackbarth C. J., Scott K. G., Rusnak M. G., Sande M. A. New cephalosporins cefotaxime, cefpimizole, BMY 28142, and HR 810 in experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1985 Mar;27(3):340–342. doi: 10.1128/aac.27.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]


