Abstract
Mutants of human rhinovirus type 2 (HRV-2) resistant to and dependent on the antirhinoviral compound chalcone Ro 09-0410 were selected in cell culture under clean laboratory conditions. A total of 42 volunteers were challenged with either the drug-resistant mutant [SR2-410(r)] (15 volunteers), the drug-dependent mutant [SR2-410(d)] (15 volunteers), or a wild-type HRV-2 which had a similar passage level in vitro as the mutants but without the drug (12 volunteers). Of volunteers challenged with the wild-type HRV-2, 33, 67, and 82% developed cold symptoms, shed virus, and showed serological evidence of infection, respectively. In contrast, only 13, 27, and 23% of volunteers challenged with the drug-resistant mutant developed colds, shed virus, and showed serological evidence of infection, respectively. None of the volunteers challenged with the drug-dependent virus became infected or had symptoms of colds. These results demonstrate that a drug-resistant rhinovirus was capable of infecting humans and producing disease, although its infectivity was reduced when compared with that of the wild type. In contrast, a drug-dependent virus had lost its ability to infect humans.
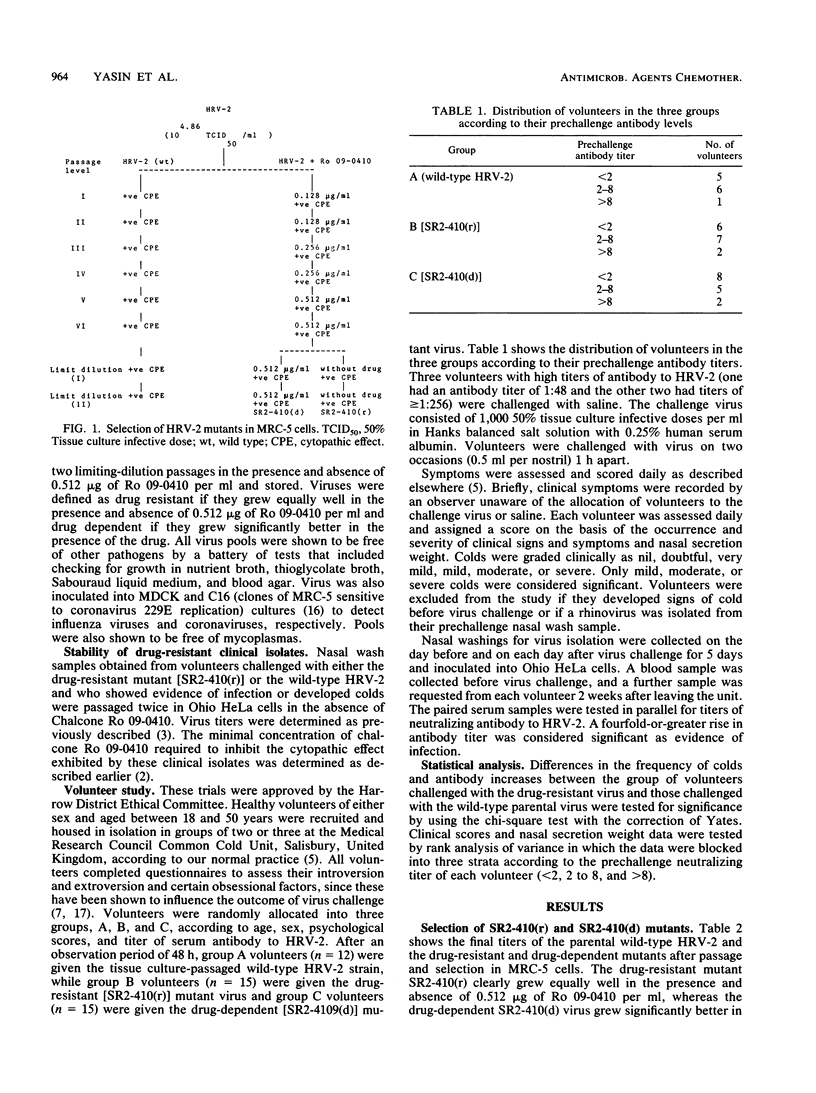
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nakib W., Tyrrell D. A. A 'new' generation of more potent synthetic antirhinovirus compounds: comparison of their MICs and their synergistic interactions. Antiviral Res. 1987 Nov;8(4):179–187. doi: 10.1016/0166-3542(87)90072-6. [DOI] [PubMed] [Google Scholar]
- Badger J., Minor I., Kremer M. J., Oliveira M. A., Smith T. J., Griffith J. P., Guerin D. M., Krishnaswamy S., Luo M., Rossmann M. G. Structural analysis of a series of antiviral agents complexed with human rhinovirus 14. Proc Natl Acad Sci U S A. 1988 May;85(10):3304–3308. doi: 10.1073/pnas.85.10.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent D. E., Broadbent M. H., Phillpotts R. J., Wallace J. Some further studies on the prediction of experimental colds in volunteers by psychological factors. J Psychosom Res. 1984;28(6):511–523. doi: 10.1016/0022-3999(84)90085-0. [DOI] [PubMed] [Google Scholar]
- Dearden C., al-Nakib W., Andries K., Woestenborghs R., Tyrrell D. A. Drug resistant rhinoviruses from the nose of experimentally treated volunteers. Arch Virol. 1989;109(1-2):71–81. doi: 10.1007/BF01310519. [DOI] [PubMed] [Google Scholar]
- Field H. J., Goldthorpe S. E. Antiviral drug resistance. Trends Pharmacol Sci. 1989 Aug;10(8):333–337. doi: 10.1016/0165-6147(89)90069-2. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Hall C. B., McBride J. T., Gala C. L., Hildreth S. W., Schnabel K. C. Ribavirin treatment of respiratory syncytial viral infection in infants with underlying cardiopulmonary disease. JAMA. 1985 Dec 6;254(21):3047–3051. [PubMed] [Google Scholar]
- Hayden F. G., Belshe R. B., Clover R. D., Hay A. J., Oakes M. G., Soo W. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N Engl J Med. 1989 Dec 21;321(25):1696–1702. doi: 10.1056/NEJM198912213212502. [DOI] [PubMed] [Google Scholar]
- Ishitsuka H., Ninomiya Y. T., Ohsawa C., Fujiu M., Suhara Y. Direct and specific inactivation of rhinovirus by chalcone Ro 09-0410. Antimicrob Agents Chemother. 1982 Oct;22(4):617–621. doi: 10.1128/aac.22.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillpotts R. J. Clones of MRC-C cells may be superior to the parent line for the culture of 229E-like strains of human respiratory coronavirus. J Virol Methods. 1983 May;6(5):267–269. doi: 10.1016/0166-0934(83)90041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totman R., Kiff J., Reed S. E., Craig J. W. Predicting experimental colds in volunteers from different measures of recent life stress. J Psychosom Res. 1980;24(3-4):155–163. doi: 10.1016/0022-3999(80)90037-9. [DOI] [PubMed] [Google Scholar]
- al-Nakib W., Higgins P. G., Barrow G. I., Tyrrell D. A., Andries K., Vanden Bussche G., Taylor N., Janssen P. A. Suppression of colds in human volunteers challenged with rhinovirus by a new synthetic drug (R61837). Antimicrob Agents Chemother. 1989 Apr;33(4):522–525. doi: 10.1128/aac.33.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]


