Abstract
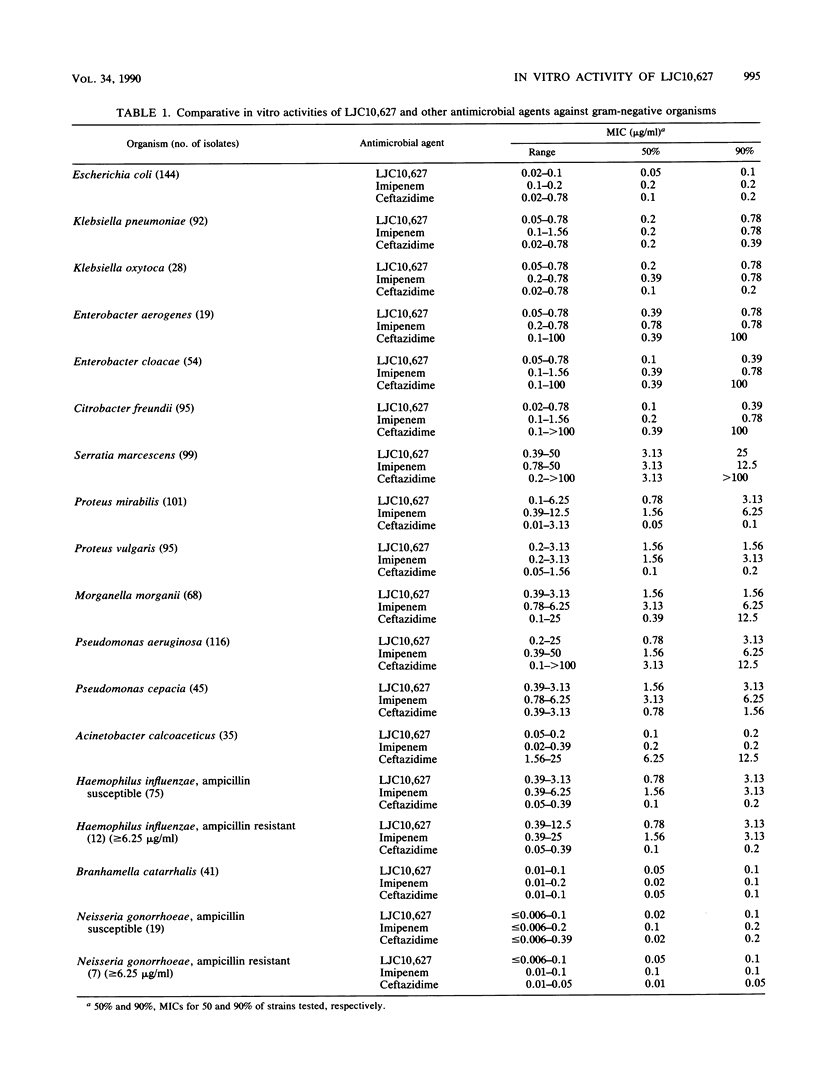
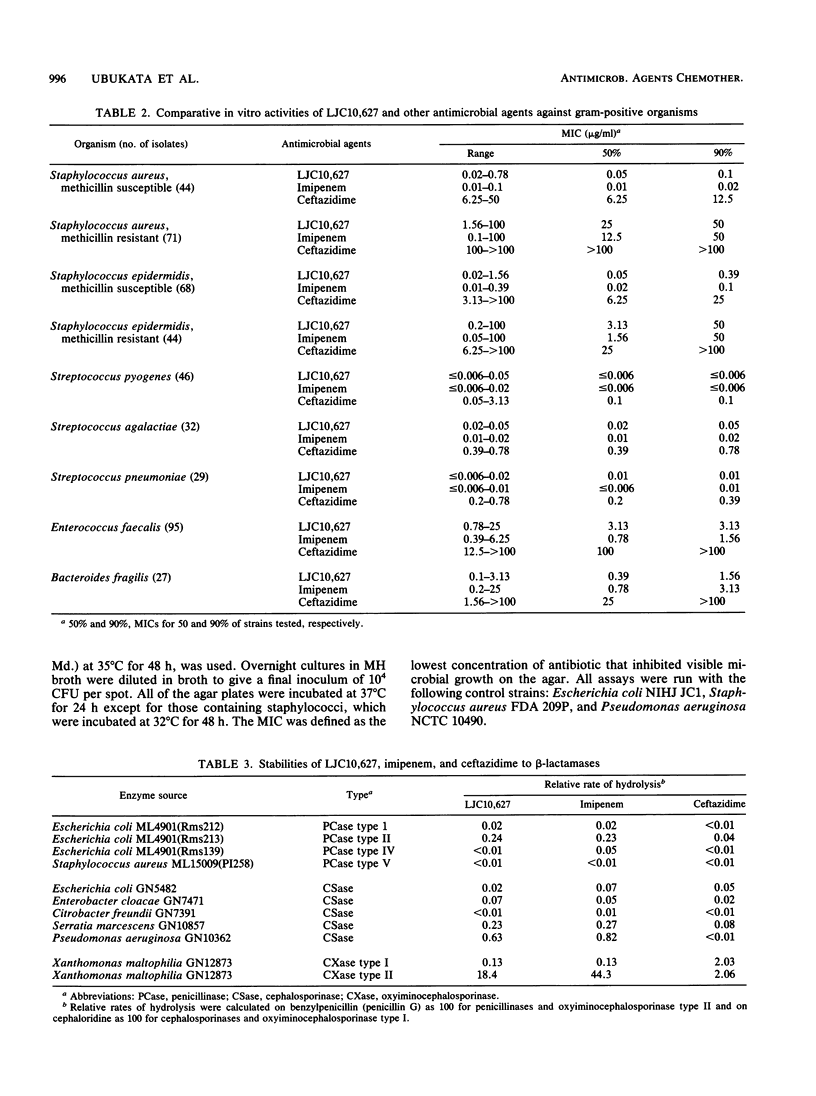
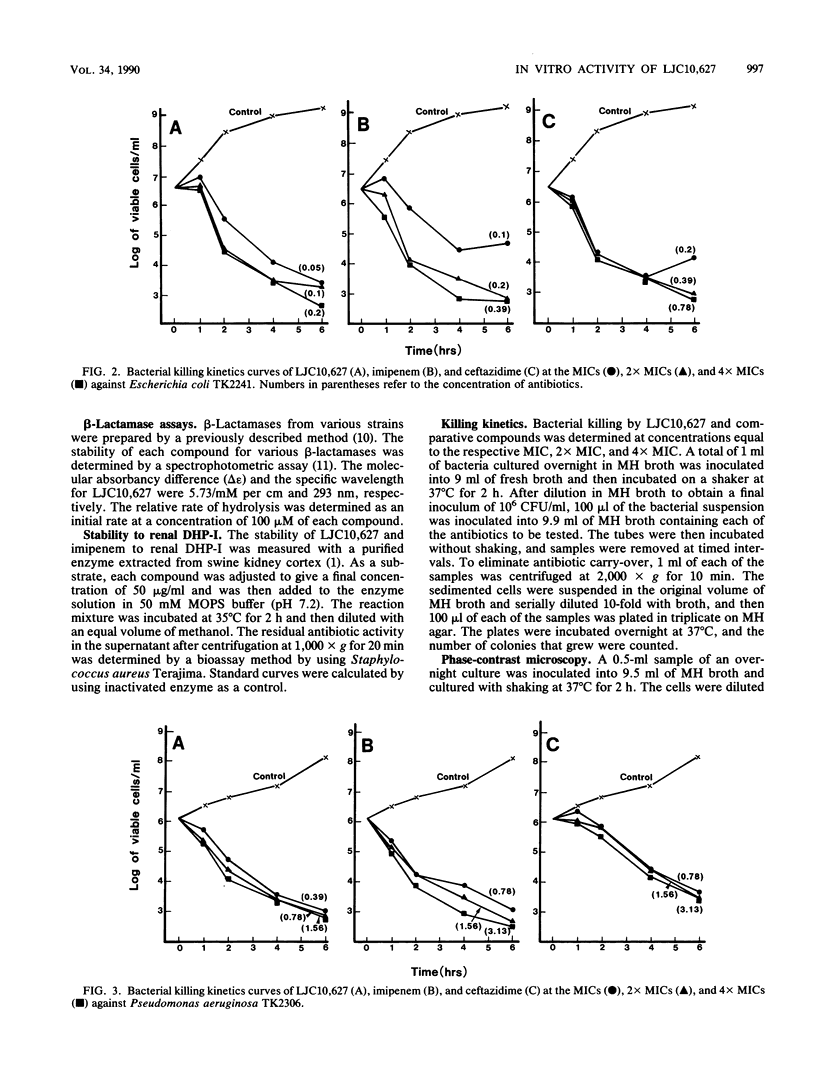
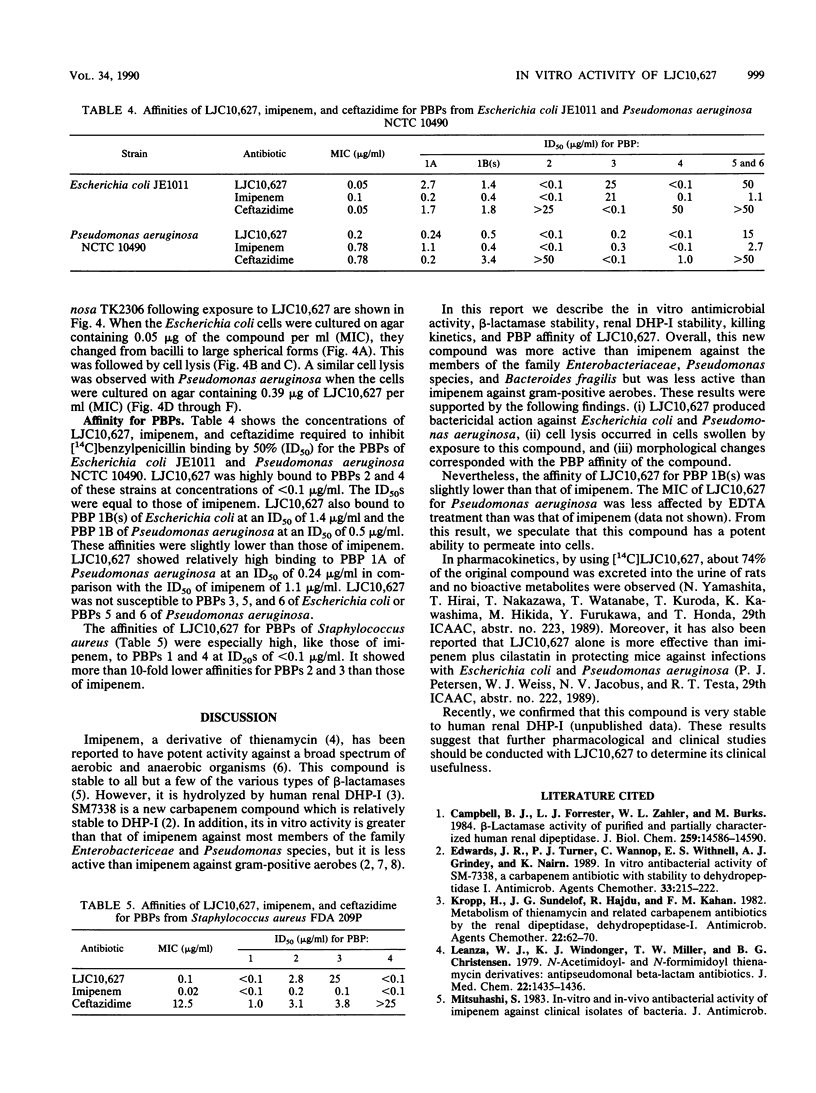
The in vitro activity of LJC10,627, a new carbapenem, was compared with those of imipenem and ceftazidime. LJC10,627 had broad-spectrum activity against gram-positive and gram-negative clinical isolates. The MICs of this compound for 90% of members of the family Enterobacteriaceae tested (MIC90s), including strains resistant to ceftazidime, ranged from 0.1 to 25 micrograms/ml. LJC10,627 inhibited Pseudomonas aeruginosa at an MIC90 of 3.13 micrograms/ml; it thus was twofold more active than imipenem. This compound inhibited Haemophilus, Neisseria, and Branhamella species at MIC90s of 3.13, 0.1, and 0.1 micrograms/ml, respectively. LJC10,627 was two- to fourfold less active than imipenem against methicillin-susceptible Staphylococcus aureus and Staphylococcus epidermidis at MIC90s of 0.1 and 0.39 microgram/ml. However, the compound was found to be twofold more active than imipenem against Bacteroides fragilis at an MIC90 of 1.56 microgram/ml. LJC10,627 was very stable to various beta-lactamases except for Xanthomonas maltophilia oxyiminocephalosporinase type II. LJC10,627 was minimally hydrolyzed by swine renal dehydropeptidase I; its residual activity was 93.0% after 2 h. Killing kinetics of this compound for Escherichia coli and Pseudomonas aeruginosa showed that bactericidal action occurred at concentrations above the MIC (0.05 and 0.39 microgram/ml, respectively). LJC10,627 had a high affinity for penicillin-binding proteins 2, 4, and 1B(s) of Escherichia coli and Pseudomonas aeruginosa and penicillin-binding proteins 1 and 4 of Staphylococcus aureus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell B. J., Forrester L. J., Zahler W. L., Burks M. Beta-lactamase activity of purified and partially characterized human renal dipeptidase. J Biol Chem. 1984 Dec 10;259(23):14586–14590. [PubMed] [Google Scholar]
- Edwards J. R., Turner P. J., Wannop C., Withnell E. S., Grindey A. J., Nairn K. In vitro antibacterial activity of SM-7338, a carbapenem antibiotic with stability to dehydropeptidase I. Antimicrob Agents Chemother. 1989 Feb;33(2):215–222. doi: 10.1128/aac.33.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Hajdu R., Kahan F. M. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase. Antimicrob Agents Chemother. 1982 Jul;22(1):62–70. doi: 10.1128/aac.22.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanza W. J., Wildonger K. J., Miller T. W., Christensen B. G. N-Acetimidoyl- and N-formimidoylthienamycin derivatives: antipseudomonal beta-lactam antibiotics. J Med Chem. 1979 Dec;22(12):1435–1436. doi: 10.1021/jm00198a001. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Comparative in vitro activity of N-formimidoyl thienamycin against gram-positive and gram-negative aerobic and anaerobic species and its beta-lactamase stability. Antimicrob Agents Chemother. 1982 Jan;21(1):180–187. doi: 10.1128/aac.21.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Novelli A., Chin N. X. In vitro activity and beta-lactamase stability of a new carbapenem, SM-7338. Antimicrob Agents Chemother. 1989 Jul;33(7):1009–1018. doi: 10.1128/aac.33.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentochnik D. E., Eliopoulos G. M., Ferraro M. J., Moellering R. C., Jr Comparative in vitro activity of SM7338, a new carbapenem antimicrobial agent. Antimicrob Agents Chemother. 1989 Aug;33(8):1232–1236. doi: 10.1128/aac.33.8.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Okamoto R., Yoshida T., Yamamoto H., Kondo S., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activities of ME1207, a new oral cephalosporin. Antimicrob Agents Chemother. 1988 Sep;32(9):1421–1426. doi: 10.1128/aac.32.9.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]