Abstract
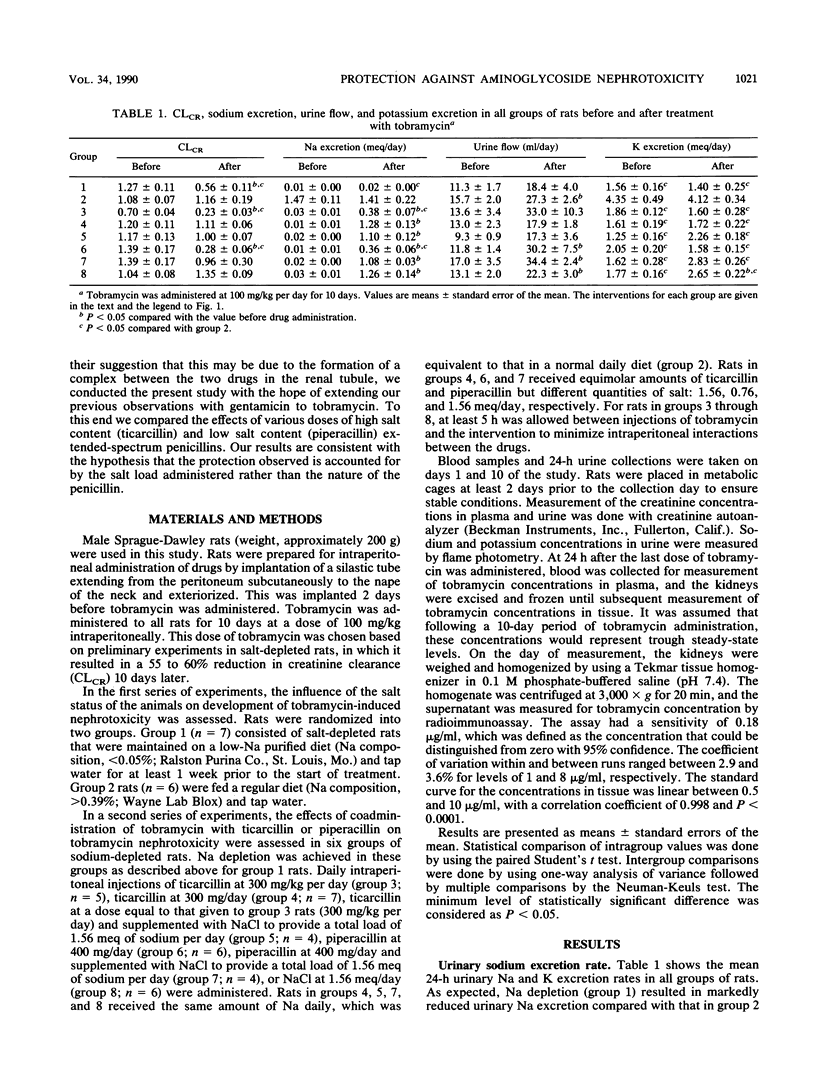
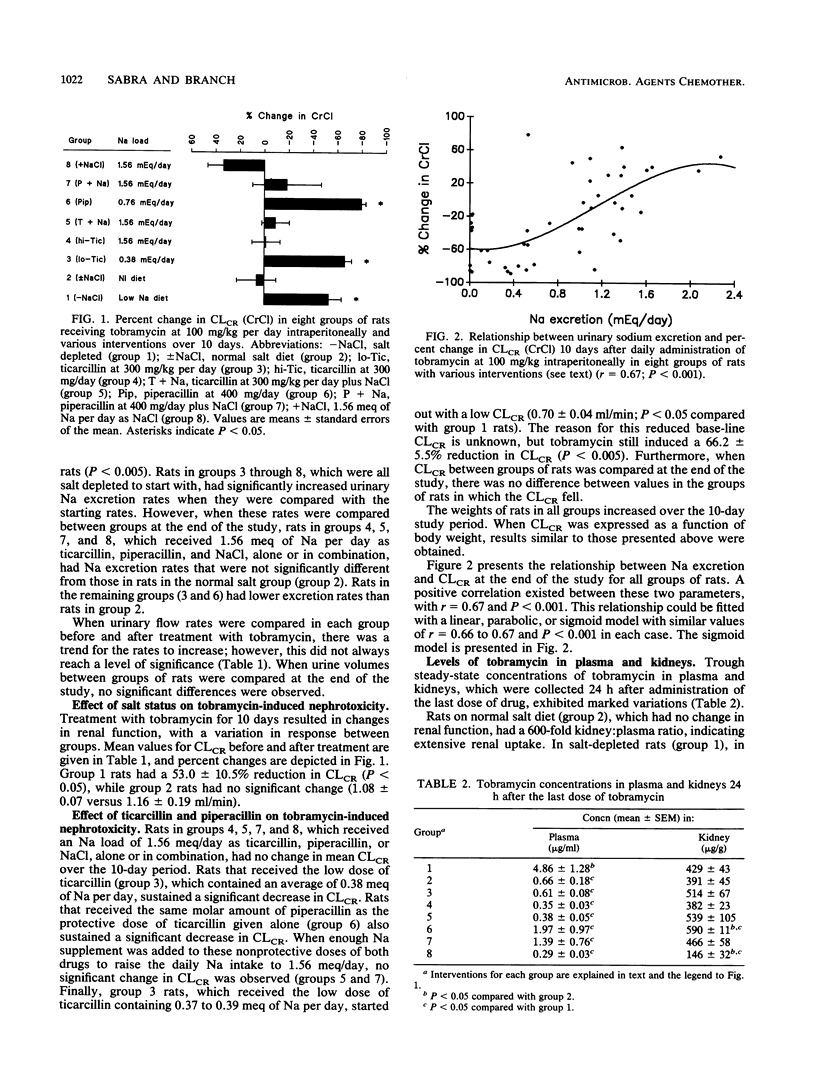
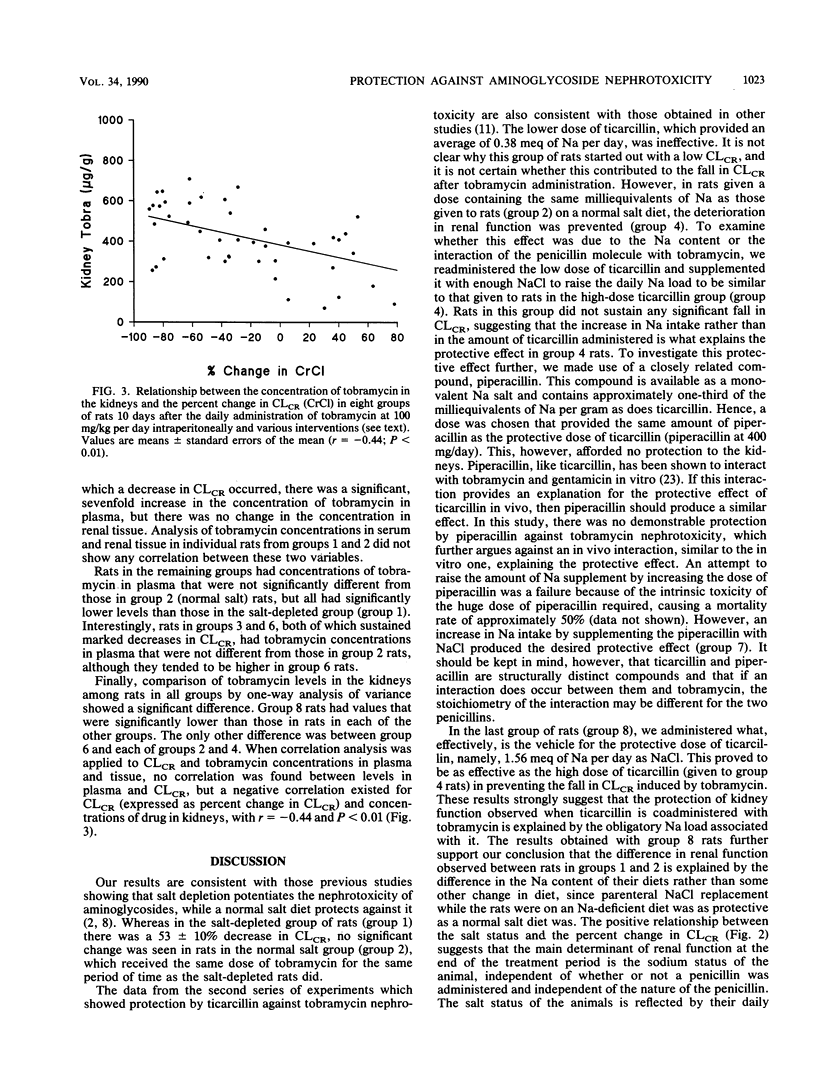
Salt depletion is known to potentiate aminoglycoside nephrotoxicity, while salt replacement attenuates it. Recent studies have shown that ticarcillin protects against tobramycin and gentamicin nephrotoxicity. It has been suggested that this protection is due to an interaction between ticarcillin and the aminoglycoside. However, it can also be explained by the salt load associated with ticarcillin administration. This study was conducted to examine this question. Tobramycin was administered to eight groups of rats at 100 mg/kg per day intraperitoneally for 10 days. Group 1 rats were salt depleted, while group 2 rats were on a normal salt diet. Rats in groups 3 through 8 were also salt depleted but received, in addition, the following interventions intraperitoneally: group 3, ticarcillin, 300 mg/kg per day (0.37 to 0.39 meq of Na supplement per day); group 4, ticarcillin, 300 mg per day (1.56 meq of Na supplement per day); group 5, ticarcillin, 300 mg/kg per day, and NaCl supplement (1.17 to 1.19 meq/day), resulting in a total load of 1.56 meq/day; group 6, piperacillin, 400 mg/day (0.76 meq of Na supplement per day and equimolar to the ticarcillin dose [300 mg/day] in group 4 rats); group 7, piperacillin, 400 mg/day, and NaCl supplement (0.8 meq/day) for a total Na load of 1.56 meq/day; and group 8, 1.56 meq of Na per day as NaCl. Rats in groups 2, 4, 5, 7, and 8, which received a normal salt diet or its equivalent Na supplement, had no significant change in creatinine clearance (CLCR) over the 10-day period. The remaining groups sustained significant reductions in CLCR, as follows: group 1, -53.0% (P < 0.05); group 3, -66.2% (P < 0.05); group 6, -79.8% (P < 0.05). A positive correlation was found between the concentration of tobramycin in the kidneys and the percent change in CLCR at the end of the study. Concentrations of drugs in plasma were highest in group 1 rats, lowest in the rats in groups in which protection was observed, and moderately elevated in the remaining groups of rats. The results of this study suggest the following: (i) that the protective effect of ticarcillin against tobramycin nephrotoxicity is secondary to the obligatory sodium load associated with it, (ii) pharmacokinetic and pharmacodynamic interactions between salt and tobramycin are proposed to explain this effect, (iii) the nephrotoxicity of tobramycin is probably related to the degree of accumulation of the drug in the kidney, and (iv) an in vivo interaction between tobramycin and ticarcillin does not contribute to the protective effect of the penicillin but may influence concentrations in plasma, especially under conditions of severe renal impairment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff G. R., Pottratz S. T., Brier M. E., Walker N. E., Fineberg N. S., Glant M. D., Luft F. C. Aminoglycoside accumulation kinetics in rat renal parenchyma. Antimicrob Agents Chemother. 1983 Jan;23(1):74–78. doi: 10.1128/aac.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W. M., Elliott W. C., Houghton D. C., Gilbert D. N., DeFehr J., McCarron D. A. Reduction of experimental gentamicin nephrotoxicity in rats by dietary calcium loading. Antimicrob Agents Chemother. 1982 Sep;22(3):508–512. doi: 10.1128/aac.22.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W. M., Hartnett M. N., Gilbert D., Houghton D., Porter G. A. Effect of sodium intake on gentamicin nephrotoxicity in the rat. Proc Soc Exp Biol Med. 1976 Apr;151(4):736–738. doi: 10.3181/00379727-151-39296. [DOI] [PubMed] [Google Scholar]
- Blair D. C., Duggan D. O., Schroeder E. T. Inactivation of amikacin and gentamicin by carbenicillin in patients with end-stage renal failure. Antimicrob Agents Chemother. 1982 Sep;22(3):376–379. doi: 10.1128/aac.22.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P. J., Long J. F. Effects of hydration on gentamicin excretion and renal accumulation in furosemide-treated rats. Antimicrob Agents Chemother. 1978 Aug;14(2):214–217. doi: 10.1128/aac.14.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M. S., Quintiliani R., Nightingale C. H. In vivo inactivation of tobramycin by ticarcillin. A case report. JAMA. 1982 Feb 5;247(5):658–659. [PubMed] [Google Scholar]
- Ebert S. C., Jorgensen J. H., Drutz D. J., Clementi W. A. Comparative assessment of in vitro inactivation of gentamicin in the presence of carbenicillin by three different gentamicin assay methods. J Clin Microbiol. 1984 Oct;20(4):701–705. doi: 10.1128/jcm.20.4.701-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. J., Schentag J. J. In vitro interactions between beta-lactam antibiotics and tobramycin. Clin Chem. 1981 Feb;27(2):341–341. [PubMed] [Google Scholar]
- English J., Gilbert D. N., Kohlhepp S., Kohnen P. W., Mayor G., Houghton D. C., Bennett W. M. Attenuation of experimental tobramycin nephrotoxicity by ticarcillin. Antimicrob Agents Chemother. 1985 Jun;27(6):897–902. doi: 10.1128/aac.27.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin F. R., Bullock W. E., Jr, Nuttall C. E. Inactivation of gentamicin by penicillins in patients with renal failure. Antimicrob Agents Chemother. 1976 Jun;9(6):1004–1011. doi: 10.1128/aac.9.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkens J. F., Branch R. A. The influence of sodium status and furosemide on canine acute amphotericin B nephrotoxicity. J Pharmacol Exp Ther. 1980 Aug;214(2):306–311. [PubMed] [Google Scholar]
- Hayashi T., Watanabe Y., Kumano K., Kitayama R., Yasuda T., Saikawa I., Katahira J., Kumada T., Shimizu K. Protective effect of piperacillin against nephrotoxicity of cephaloridine and gentamicin in animals. Antimicrob Agents Chemother. 1988 Jun;32(6):912–918. doi: 10.1128/aac.32.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf-Leurs M. M., Ladik T., Smolka B., Bock T., Schramm W., Spannagl M., Landgraf R. Increased thromboplastic potential in diabetes: a multifactorial phenomenon. Klin Wochenschr. 1987 Jul 1;65(13):600–606. doi: 10.1007/BF01726667. [DOI] [PubMed] [Google Scholar]
- Lecompte J., Dumont L., Hill J., du Souich P., Lelorier J. Effect of water deprivation and rehydration on gentamicin disposition in the rat. J Pharmacol Exp Ther. 1981 Jul;218(1):231–236. [PubMed] [Google Scholar]
- Luft F. C., Aronoff G. R., Evan A. P., Connors B. A., Weinberger M. H., Kleit S. A. The renin-angiotensin system in aminoglycoside-induced acute renal failure. J Pharmacol Exp Ther. 1982 Feb;220(2):433–439. [PubMed] [Google Scholar]
- McLaughlin J. E., Reeves D. S. Clinical and laboratory evidence for inactivation of gentamicin by carbenicillin. Lancet. 1971 Feb 6;1(7693):261–264. doi: 10.1016/s0140-6736(71)91001-4. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Weinstock L. B., Gump D. W., Hacker M. P., Yates J. W. Effect of osmotic diuresis on gentamicin-induced nephrotoxicity in rats. Arch Toxicol. 1980 Sep;45(3):213–221. doi: 10.1007/BF02419001. [DOI] [PubMed] [Google Scholar]
- Ohnishi A., Bryant T. D., Branch K. R., Sabra R., Branch R. A. Role of sodium in the protective effect of ticarcillin on gentamicin nephrotoxicity in rats. Antimicrob Agents Chemother. 1989 Jun;33(6):928–932. doi: 10.1128/aac.33.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osswald H., Hermes H. H., Nabakowski G. Role of adenosine in signal transmission of tubuloglomerular feedback. Kidney Int Suppl. 1982 Aug;12:S136–S142. [PubMed] [Google Scholar]
- Pickering L. K., Gearhart P. Effect of time and concentration upon interaction between gentamicin, tobramycin, Netilmicin, or amikacin and carbenicillin or ticarcillin. Antimicrob Agents Chemother. 1979 Apr;15(4):592–596. doi: 10.1128/aac.15.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering L. K., Rutherford I. Effect of concentration and time upon inactivation of tobramycin, gentamicin, netilmicin and amikacin by azlocillin, carbenicillin, mecillinam, mezlocillin and piperacillin. J Pharmacol Exp Ther. 1981 May;217(2):345–349. [PubMed] [Google Scholar]
- Schor N., Ichikawa I., Rennke H. G., Troy J. L., Brenner B. M. Pathophysiology of altered glomerular function in aminoglycoside-treated rats. Kidney Int. 1981 Feb;19(2):288–296. doi: 10.1038/ki.1981.19. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Wiernik P. H. Antibiotic combination-associated nephrotoxicity in granulocytopenic patients with cancer. Arch Intern Med. 1981 Dec;141(13):1789–1793. doi: 10.1001/archinte.141.13.1789. [DOI] [PubMed] [Google Scholar]
- Wallace S. M., Chan L. Y. In vitro interaction of aminoglycosides with beta-lactam penicillins. Antimicrob Agents Chemother. 1985 Aug;28(2):274–281. doi: 10.1128/aac.28.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rougemont D., Oeschger A., Konrad L., Thiel G., Torhorst J., Wenk M., Wunderlich P., Brunner F. P. Gentamicin-induced acute renal failure in the rat. Effect of dehydration, DOCA-saline and furosemide. Nephron. 1981;29(3-4):176–184. doi: 10.1159/000182352. [DOI] [PubMed] [Google Scholar]


