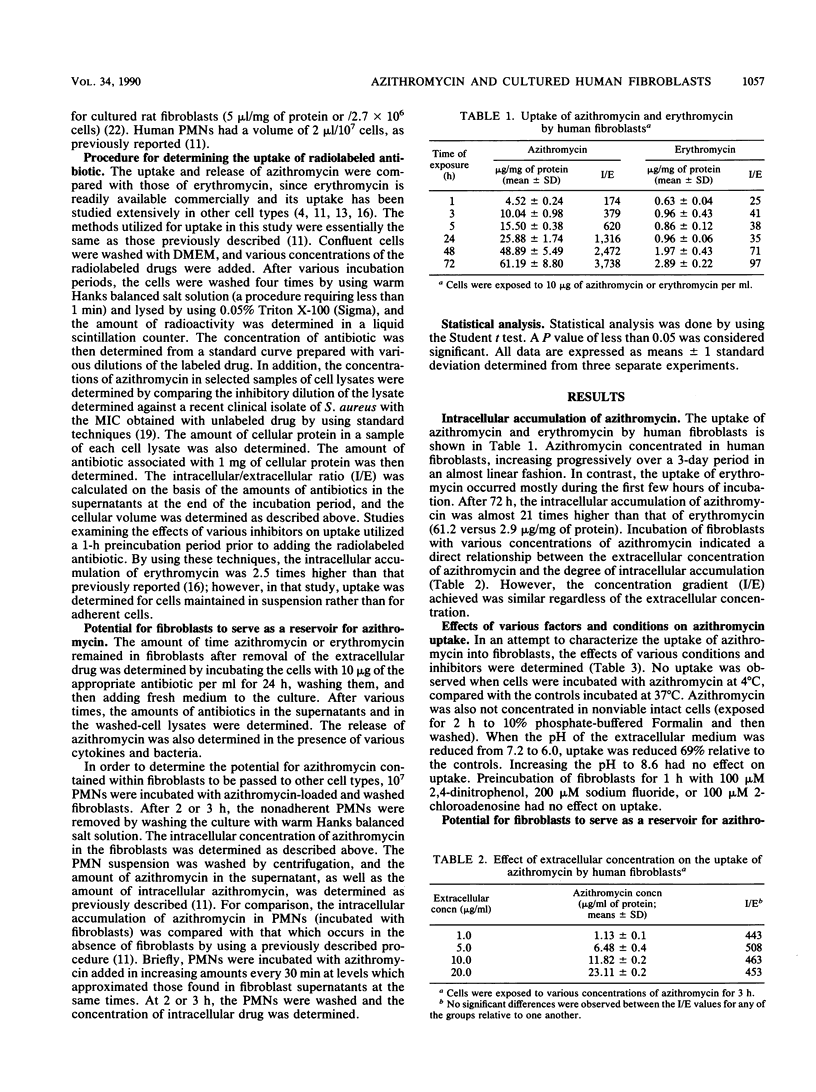
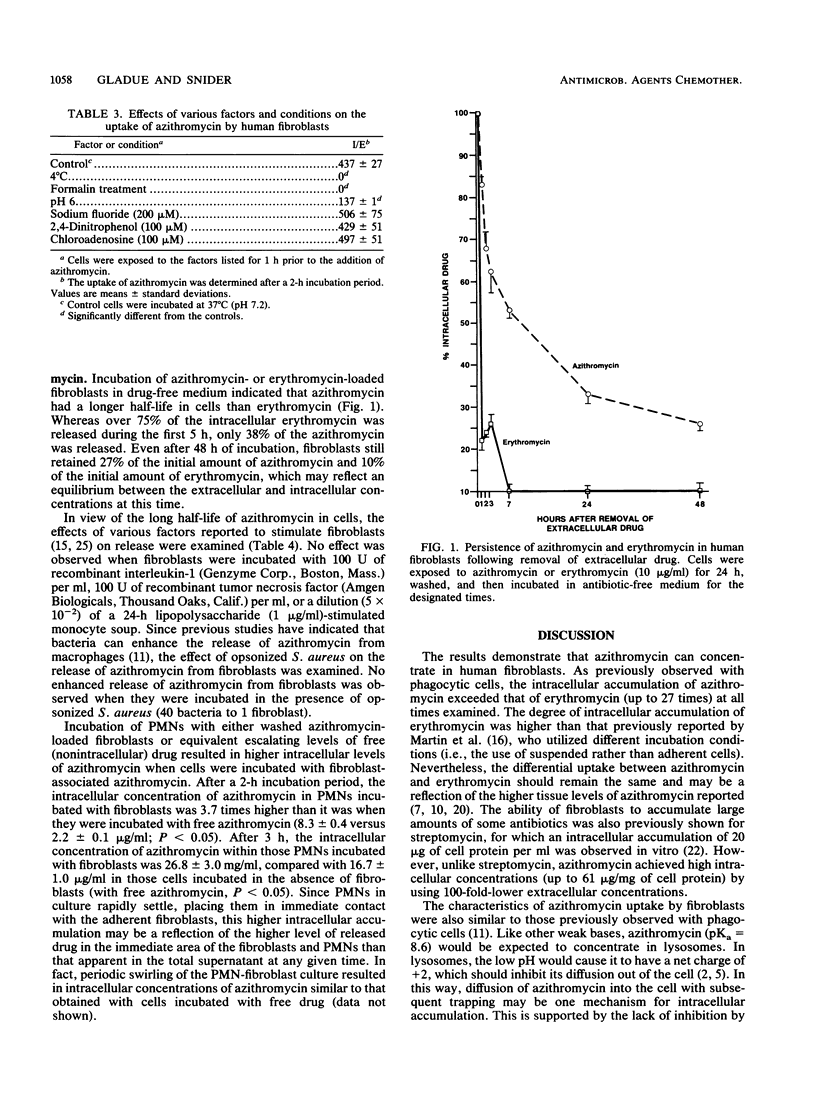
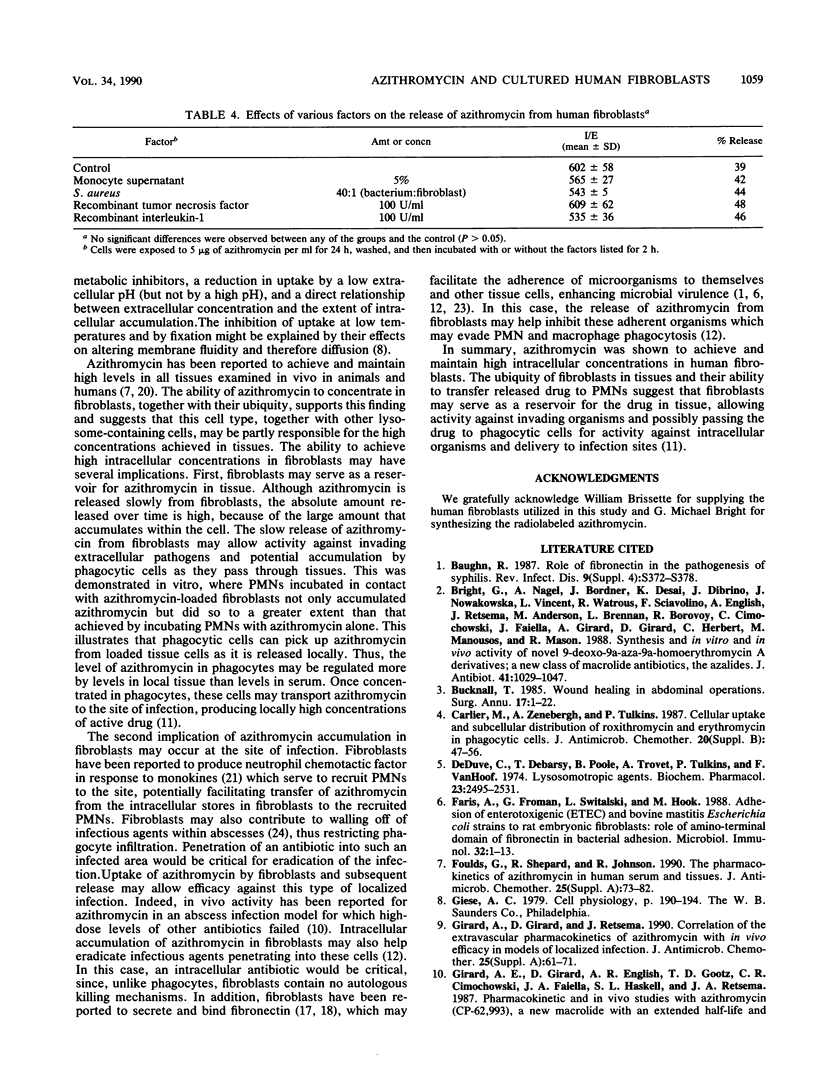
Abstract
Azithromycin was shown to achieve high concentrations in human skin fibroblasts. Intracellular penetration occurred rapidly (10 micrograms/mg of cellular protein after 3 h) and then increased progressively over a 3-day period; azithromycin accumulated up to 21 times more than erythromycin (61.1 versus 2.9 micrograms/mg of protein). Uptake was dependent on the extracellular concentration, was inhibited at 4 degrees C, did not occur in nonviable cells, and was reduced by a low pH. Intracellular accumulation was not affected by the metabolic inhibitor 2,4-dinitrophenol or sodium fluoride or by the nucleoside transport inhibitor 2-chloradenosine. Once concentrated in cells, azithromycin remained intracellular and was released slowly in the absence of extracellular drug, compared with erythromycin (17 versus 78% released after 1 h). After 48 h of incubation in drug-free medium, 27% of the initial amount of azithromycin remained cell associated. The release of azithromycin was not affected by various monokines reported to stimulate fibroblasts (interleukin-1 or tumor necrosis factor) or by exposure to bacteria. Incubation of azithromycin-loaded fibroblasts with human polymorphonuclear leukocytes resulted in a higher intracellular accumulation of azithromycin in polymorphonuclear leukocytes than in cells incubated with free nonintracellular azithromycin for the same time (8.3 versus 2.2 micrograms/ml after 2 h), suggesting a more efficient or rapid uptake through cell-to-cell interaction. The widespread distribution of fibroblasts in tissues suggests a potential for these cells, and possibly other lysosome-containing tissue cells, to serve as a reservoir for azithromycin, slowly releasing it for activity against extracellular organisms at sites of infection and passing it to phagocytes for activity against intracellular pathogens and potential transport to sites of infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughn R. E. Role of fibronectin in the pathogenesis of syphilis. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S372–S385. doi: 10.1093/clinids/9.supplement_4.s372. [DOI] [PubMed] [Google Scholar]
- Bright G. M., Nagel A. A., Bordner J., Desai K. A., Dibrino J. N., Nowakowska J., Vincent L., Watrous R. M., Sciavolino F. C., English A. R. Synthesis, in vitro and in vivo activity of novel 9-deoxo-9a-AZA-9a-homoerythromycin A derivatives; a new class of macrolide antibiotics, the azalides. J Antibiot (Tokyo) 1988 Aug;41(8):1029–1047. doi: 10.7164/antibiotics.41.1029. [DOI] [PubMed] [Google Scholar]
- Bucknall T. E. Wound healing in abdominal operations. Surg Annu. 1985;17:1–22. [PubMed] [Google Scholar]
- Carlier M. B., Zenebergh A., Tulkens P. M. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):47–56. doi: 10.1093/jac/20.suppl_b.47. [DOI] [PubMed] [Google Scholar]
- Faris A., Fröman G., Switalski L., Hök M. Adhesion of enterotoxigenic (ETEC) and bovine mastitis Escherichia coli strains to rat embryonic fibroblasts: role of amino-terminal domain of fibronectin in bacterial adhesion. Microbiol Immunol. 1988;32(1):1–13. doi: 10.1111/j.1348-0421.1988.tb01360.x. [DOI] [PubMed] [Google Scholar]
- Foulds G., Shepard R. M., Johnson R. B. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., Retsema J. A. Correlation of the extravascular pharmacokinetics of azithromycin with in-vivo efficacy in models of localized infection. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):61–71. doi: 10.1093/jac/25.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- Gladue R. P., Bright G. M., Isaacson R. E., Newborg M. F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989 Mar;33(3):277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill R. J. Role of fibronectin in infective endocarditis. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S360–S371. doi: 10.1093/clinids/9.supplement_4.s360. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Kanzler M. H., Gorsulowsky D. C., Swanson N. A. Basic mechanisms in the healing cutaneous wound. J Dermatol Surg Oncol. 1986 Nov;12(11):1156–1164. doi: 10.1111/j.1524-4725.1986.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. R., Johnson P., Miller M. F. Uptake, accumulation, and egress of erythromycin by tissue culture cells of human origin. Antimicrob Agents Chemother. 1985 Mar;27(3):314–319. doi: 10.1128/aac.27.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo P. J. Fibronectin-cell surface interactions. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S322–S334. doi: 10.1093/clinids/9.supplement_4.s322. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard R. M., Falkner F. C. Pharmacokinetics of azithromycin in rats and dogs. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):49–60. doi: 10.1093/jac/25.suppl_a.49. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Tulkens P., Trouet A. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem Pharmacol. 1978 Feb 15;27(4):415–424. doi: 10.1016/0006-2952(78)90370-2. [DOI] [PubMed] [Google Scholar]
- Yildizhan A., Paşaoğlu A., Kandemir B. Effect of dexamethasone on various stages of experimental brain abscess. Acta Neurochir (Wien) 1989;96(3-4):141–148. doi: 10.1007/BF01456174. [DOI] [PubMed] [Google Scholar]
- Zucali J. R., Dinarello C. A., Oblon D. J., Gross M. A., Anderson L., Weiner R. S. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- van der Sluis J. J., Koehorst J. A., Boer A. M. Factors that inhibit adherence of Treponema pallidum (Nichols strain) to a human fibroblastic cell line: development in serum of patients with syphilis. Genitourin Med. 1987 Apr;63(2):71–76. doi: 10.1136/sti.63.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]


