Abstract
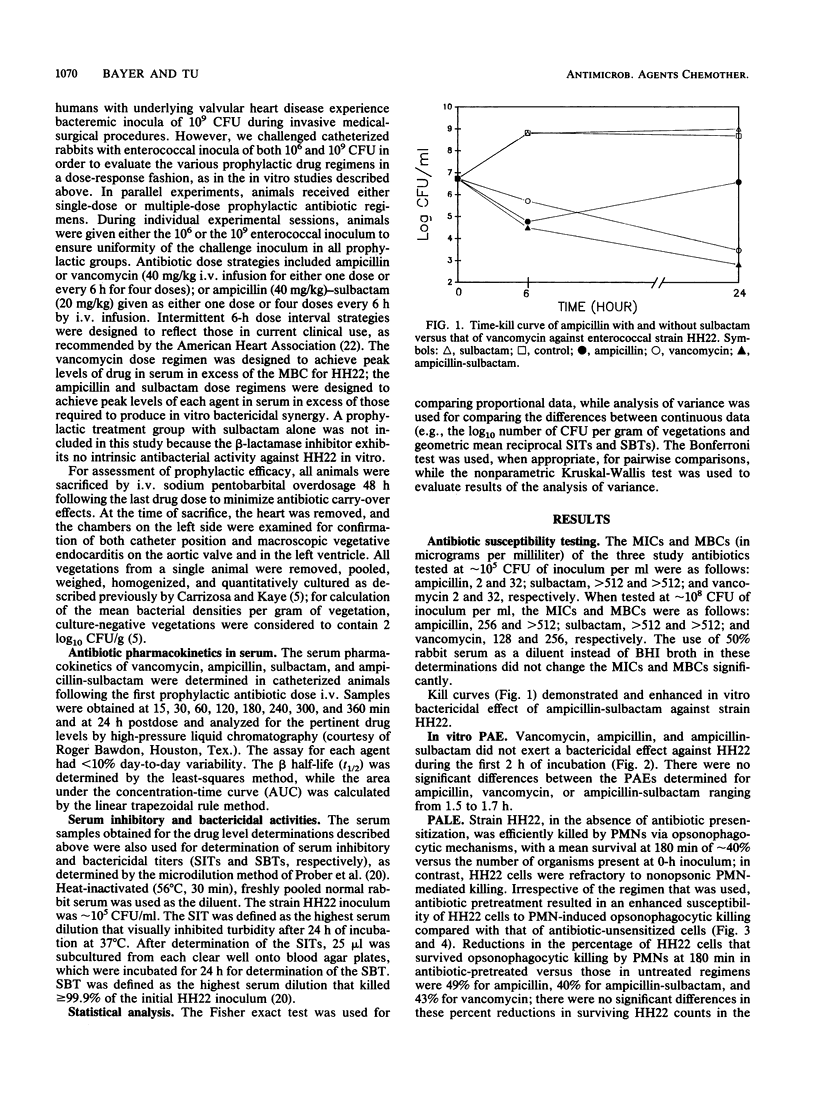
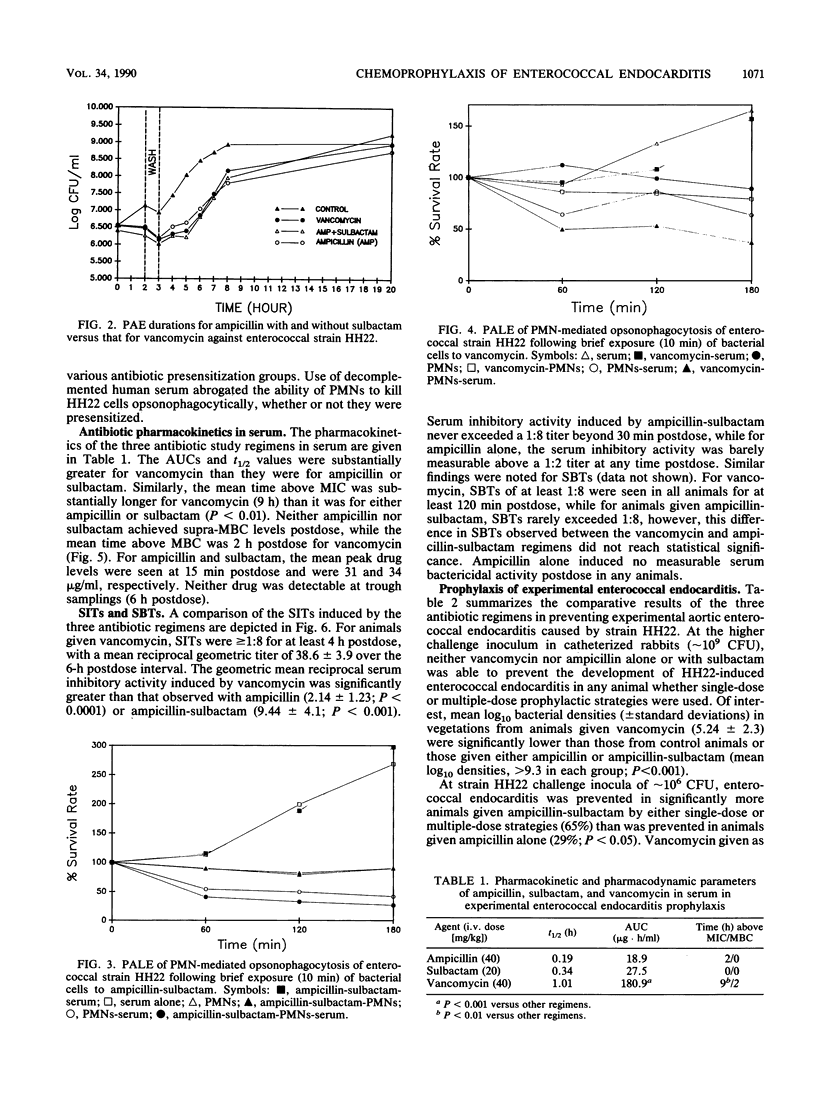
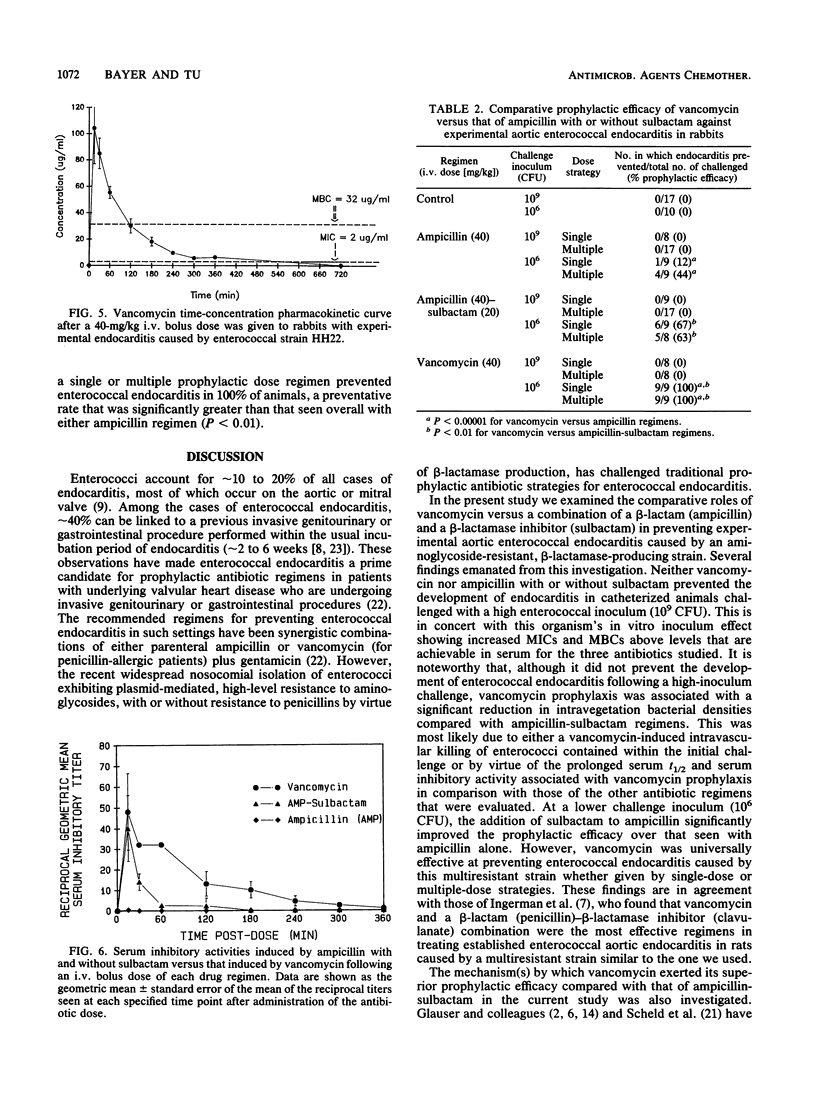
We studied the prevention of experimental aortic endocarditis caused by a beta-lactamase-producing, aminoglycoside-resistant strain of Enterococcus faecalis (HH22) in 146 catheterized rabbits. Both vancomycin and ampicillin-sulbactam readily killed this resistant enterococcus strain in vitro. At a challenge inoculum of approximately 10(9) CFU, vancomycin (40 mg/kg intravenously [i.v.]), ampicillin (40 mg/kg i.v.), or a combination of ampicillin plus a beta-lactamase inhibitor, sulbactam (20 mg/kg, i.v.), did not prevent the development of endocarditis in any of the animals, although mean intravegetation bacterial densities were significantly lower in animals that received vancomycin than they were in animals that received other therapies (P less than 0.001). At a challenge inoculum of 10(6) CFU, vancomycin was 100% effective in preventing enterococcal endocarditis compared with ampicillin (29%; P less than 0.00001) and ampicillin-sulbactam (65%; P less than 0.01). Factors associated with the superior prophylactic efficacy of vancomycin in this model included prolonged serum inhibitory activity and time above MICs. Factors not associated with the antienterococcal prophylactic efficacy of vancomycin included the duration of the in vitro postantibiotic effect of the drug and the magnitude of the ability of this drug to enhance enterococcal in vitro opsonophagocytic killing by polymorphonuclear leukocytes. The superior prophylactic efficacy of vancomycin in this endocarditis model related to the superior pharmacokinetic profile of the drug when it was given intermittently at dose intervals of every 6 h.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer A. S., Greenberg D. P., Yih J. Correlates of therapeutic efficacy in experimental methicillin-resistant Staphylococcus aureus endocarditis. Chemotherapy. 1988;34(1):46–55. doi: 10.1159/000238547. [DOI] [PubMed] [Google Scholar]
- Bernard J. P., Francioli P., Glauser M. P. Vancomycin prophylaxis of experimental Streptococcus sanguis. Inhibition of bacterial adherence rather than bacterial killing. J Clin Invest. 1981 Oct;68(4):1113–1116. doi: 10.1172/JCI110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney P., Francioli P. Successful prophylaxis of experimental streptococcal endocarditis with single-dose amoxicillin administered after bacterial challenge. J Infect Dis. 1990 Feb;161(2):281–285. doi: 10.1093/infdis/161.2.281. [DOI] [PubMed] [Google Scholar]
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizosa J., Kaye D. Antibiotic synergism in enterococcal endocarditis. J Lab Clin Med. 1976 Jul;88(1):132–141. [PubMed] [Google Scholar]
- Glauser M. P., Bernard J. P., Moreillon P., Francioli P. Successful single-dose amoxicillin prophylaxis against experimental streptococcal endocarditis: evidence for two mechanisms of protection. J Infect Dis. 1983 Mar;147(3):568–575. doi: 10.1093/infdis/147.3.568. [DOI] [PubMed] [Google Scholar]
- Ingerman M., Pitsakis P. G., Rosenberg A., Hessen M. T., Abrutyn E., Murray B. E., Levison M. E. beta-Lactamase production in experimental endocarditis due to aminoglycoside-resistant Streptococcus faecalis. J Infect Dis. 1987 Jun;155(6):1226–1232. doi: 10.1093/infdis/155.6.1226. [DOI] [PubMed] [Google Scholar]
- Kaye D. Changing pattern of infective endocarditis. Am J Med. 1985 Jun 28;78(6B):157–162. doi: 10.1016/0002-9343(85)90378-x. [DOI] [PubMed] [Google Scholar]
- Kaye D. Prophylaxis for infective endocarditis: an update. Ann Intern Med. 1986 Mar;104(3):419–423. doi: 10.7326/0003-4819-104-3-419. [DOI] [PubMed] [Google Scholar]
- McDonald P. J., Craig W. A., Kunin C. M. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977 Feb;135(2):217–223. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- McDonald P. J., Wetherall B. L., Pruul H. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev Infect Dis. 1981 Jan-Feb;3(1):38–44. doi: 10.1093/clinids/3.1.38. [DOI] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Moreillon P., Francioli P., Overholser D., Meylan P., Glauser M. P. Mechanisms of successful amoxicillin prophylaxis of experimental endocarditis due to Streptococcus intermedius. J Infect Dis. 1986 Nov;154(5):801–807. doi: 10.1093/infdis/154.5.801. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Church D. A., Wanger A., Zscheck K., Levison M. E., Ingerman M. J., Abrutyn E., Mederski-Samoraj B. Comparison of two beta-lactamase-producing strains of Streptococcus faecalis. Antimicrob Agents Chemother. 1986 Dec;30(6):861–864. doi: 10.1128/aac.30.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samoraj B., Foster S. K., Brunton J. L., Harford P. In vitro studies of plasmid-mediated penicillinase from Streptococcus faecalis suggest a staphylococcal origin. J Clin Invest. 1986 Jan;77(1):289–293. doi: 10.1172/JCI112289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Colodny S. M., Zervos M. J. Serious infection due to beta-lactamase-producing Streptococcus faecalis with high-level resistance to gentamicin. J Infect Dis. 1988 Nov;158(5):1144–1145. doi: 10.1093/infdis/158.5.1144. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman B. B., Freedman L. R. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971 Oct;44(2):206–213. [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Zak O., Vosbeck K., Sande M. A. Bacterial adhesion in the pathogenesis of infective endocarditis. Effect of subinhibitory antibiotic concentrations on streptococcal adhesion in vitro and the development of endocarditis in rabbits. J Clin Invest. 1981 Nov;68(5):1381–1384. doi: 10.1172/JCI110388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman S. T., Amren D. P., Bisno A. L., Dajani A. S., Durack D. T., Gerber M. A., Kaplan E. L., Millard H. D., Sanders W. E., Schwartz R. H. Prevention of Bacterial Endocarditis. A statement for health professionals by the Committee on Rheumatic Fever and Infective Endocarditis of the Council on Cardiovascular Disease in the Young. Circulation. 1984 Dec;70(6):1123A–1127A. [PubMed] [Google Scholar]
- Starkebaum M., Durack D., Beeson P. The "incubation period" of subacute bacterial endocarditis. Yale J Biol Med. 1977 Jan-Feb;50(1):49–58. [PMC free article] [PubMed] [Google Scholar]


