Abstract
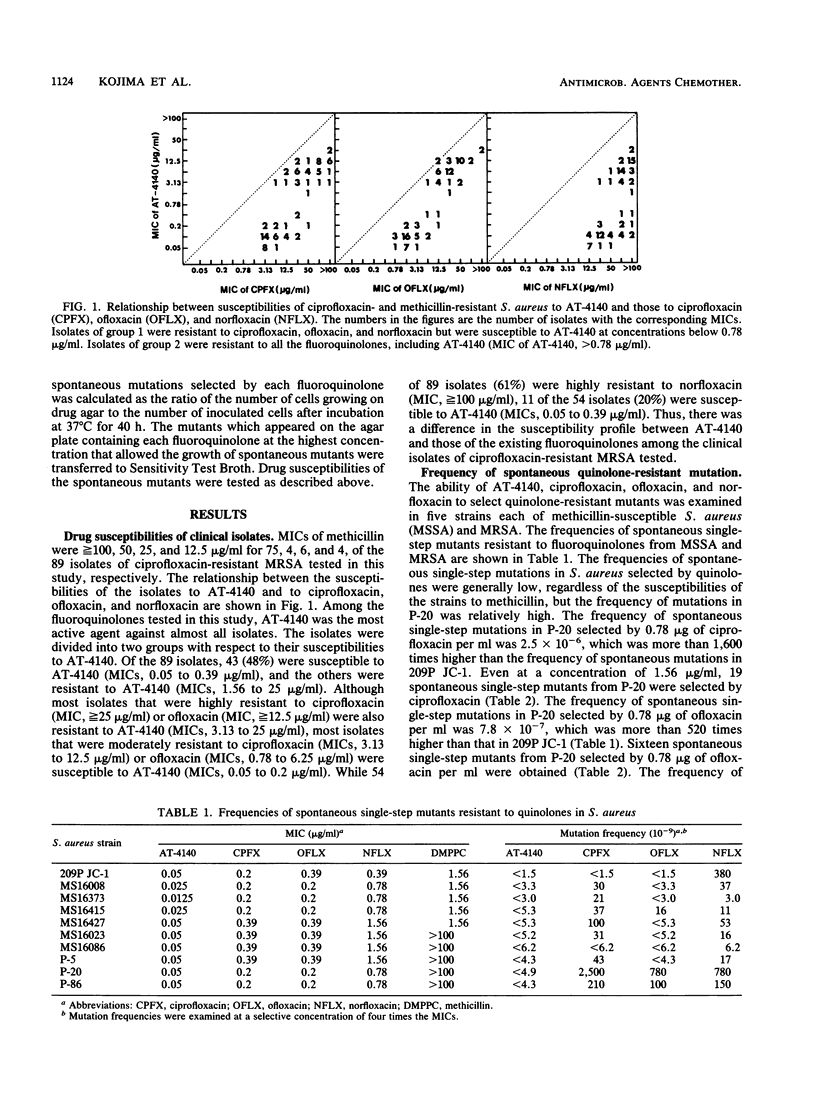
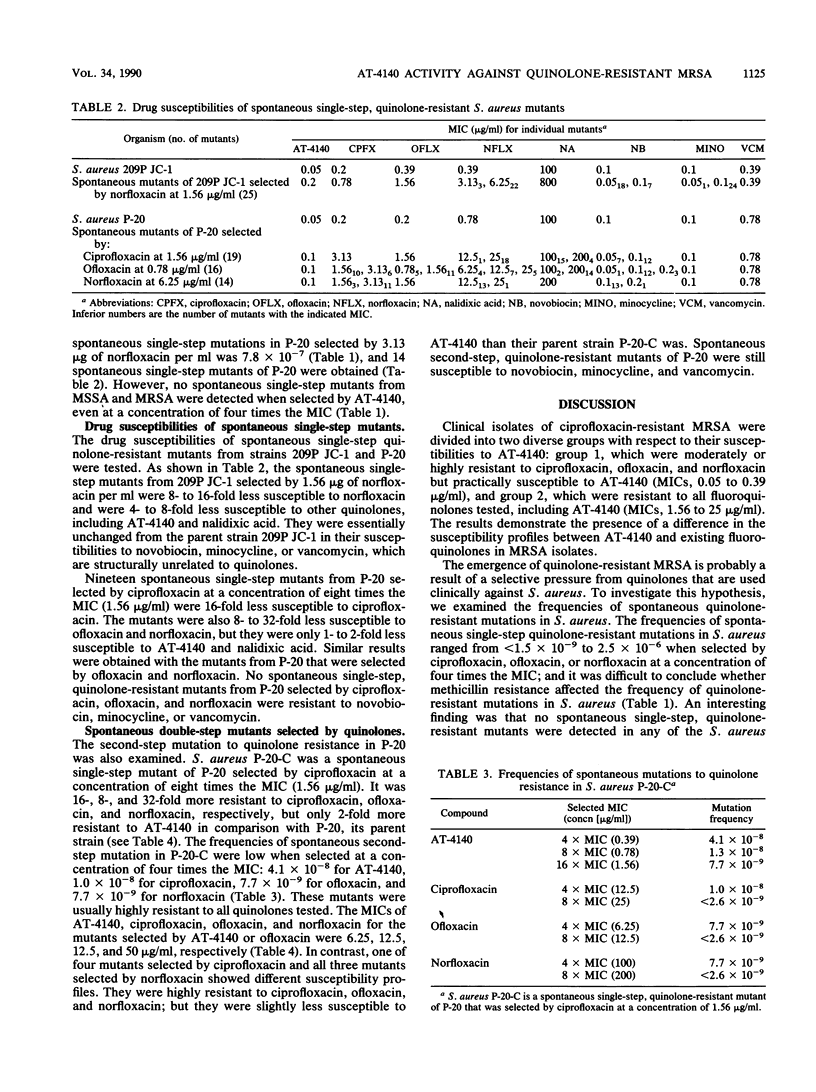
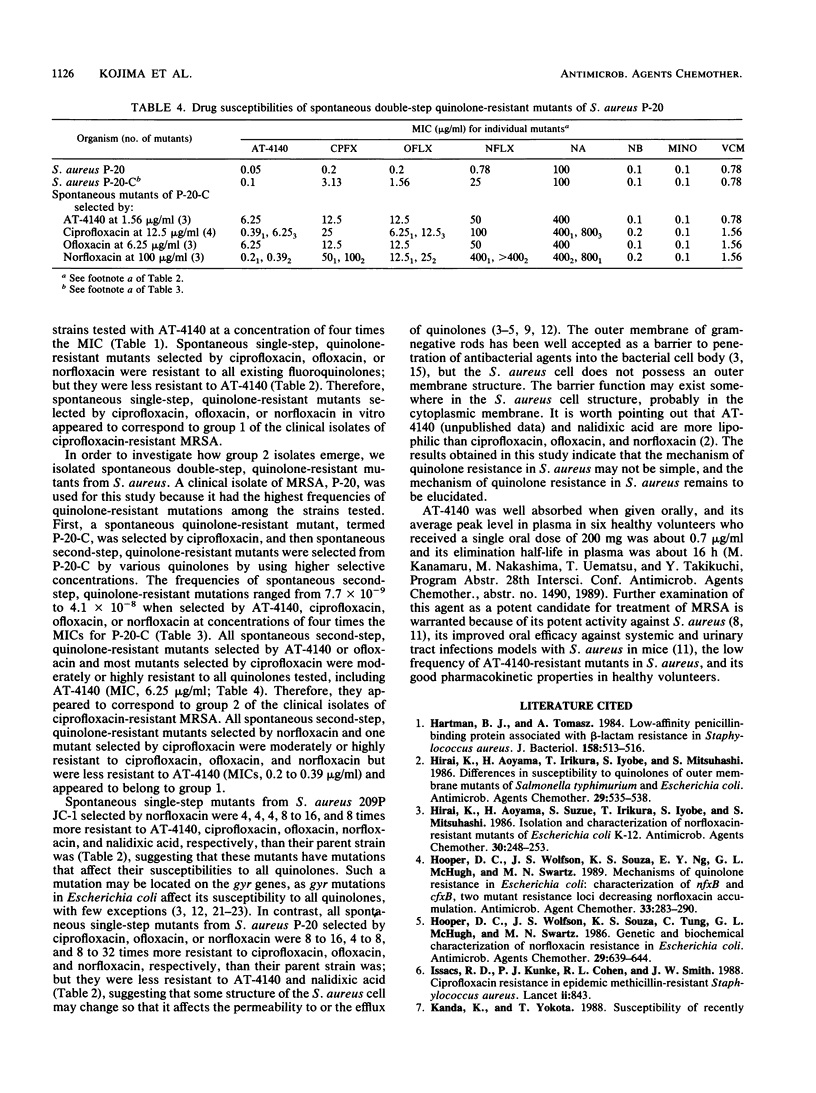
Eighty-nine clinical isolates of Staphylococcus aureus that were resistant to both ciprofloxacin (MIC, greater than or equal to 3.13 micrograms/ml) and methicillin (MIC, greater than or equal to 12.5 micrograms/ml) were divided into two groups with respect to their susceptibilities to AT-4140. Most isolates that were moderately resistant to ciprofloxacin (MICs, 3.13 to 12.5 micrograms/ml) or ofloxacin (MICs, 0.78 to 6.25 micrograms/ml) were susceptible to AT-4140 (MICs, 0.05 to 0.2 microgram/ml). Most isolates that were highly resistant to ciprofloxacin (MIC, greater than or equal to 25 micrograms/ml) or ofloxacin (MIC, greater than or equal to 12.5 micrograms/ml) were resistant to AT-4140 (MICs, 3.13 to 25 micrograms/ml). The appearance of spontaneous single-step, quinolone-resistant mutants of S. aureus P-20, a methicillin-resistant isolate, was more frequent than was that of S. aureus 209P JC-1, a susceptible laboratory strain. Spontaneous single-step, quinolone-resistant mutants of P-20 were not selected by AT-4140, and those selected by existing fluoroquinolones were susceptible to AT-4140. Spontaneous double-step, quinolone-resistant mutants of P-20 were selected by various fluoroquinolones. All second-step mutants selected by AT-4140 or ofloxacin from P-20-C, a spontaneous single-step mutant of P-20 selected by ciprofloxacin, were resistant to all the quinolones. All second-step mutants selected by nonfloxacin were resistant to all existing fluoroquinolones but were less resistant to AT-4140. There was a close resemblance between the resistance profiles of spontaneous quinolone-resistant mutants and those of clinically isolated quinolone- and methicillin-resistant S. aureus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Inoue M., Mitsuhashi S. In vitro activity of AT-4140 against clinical bacterial isolates. Antimicrob Agents Chemother. 1989 Nov;33(11):1980–1988. doi: 10.1128/aac.33.11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Minami A., Nakata K., Kurobe N., Kouno K., Sakaguchi Y., Kashimoto S., Yoshida H., Kojima T., Ohue T. In vitro and in vivo antibacterial activities of AT-4140, a new broad-spectrum quinolone. Antimicrob Agents Chemother. 1989 Aug;33(8):1167–1173. doi: 10.1128/aac.33.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Nakamura M., Kojima T., Yoshida H. gyrA and gyrB mutations in quinolone-resistant strains of Escherichia coli. Antimicrob Agents Chemother. 1989 Feb;33(2):254–255. doi: 10.1128/aac.33.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy E. A., Barbaro D., Luby J. P., Mackowiak P. A. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 1989 Jan;33(1):128–130. doi: 10.1128/aac.33.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C. Ciprofloxacin: in vitro activity, mechanism of action, and resistance. Rev Infect Dis. 1988 May-Jun;10(3):516–527. doi: 10.1093/clinids/10.3.516. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H. Activity of ciprofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):688–691. doi: 10.1128/aac.27.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Tecson-Tumang F. Ciprofloxacin therapy for methicillin-resistant Staphylococcus aureus infections or colonizations. Antimicrob Agents Chemother. 1989 Feb;33(2):181–184. doi: 10.1128/aac.33.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Furutani Y., Inoue S., Ohue T., Nakamura S., Shimizu M. New nalidixic acid resistance mutations related to deoxyribonucleic acid gyrase activity. J Bacteriol. 1981 Nov;148(2):450–458. doi: 10.1128/jb.148.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Kojima T., Yamagishi J., Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]


