Abstract
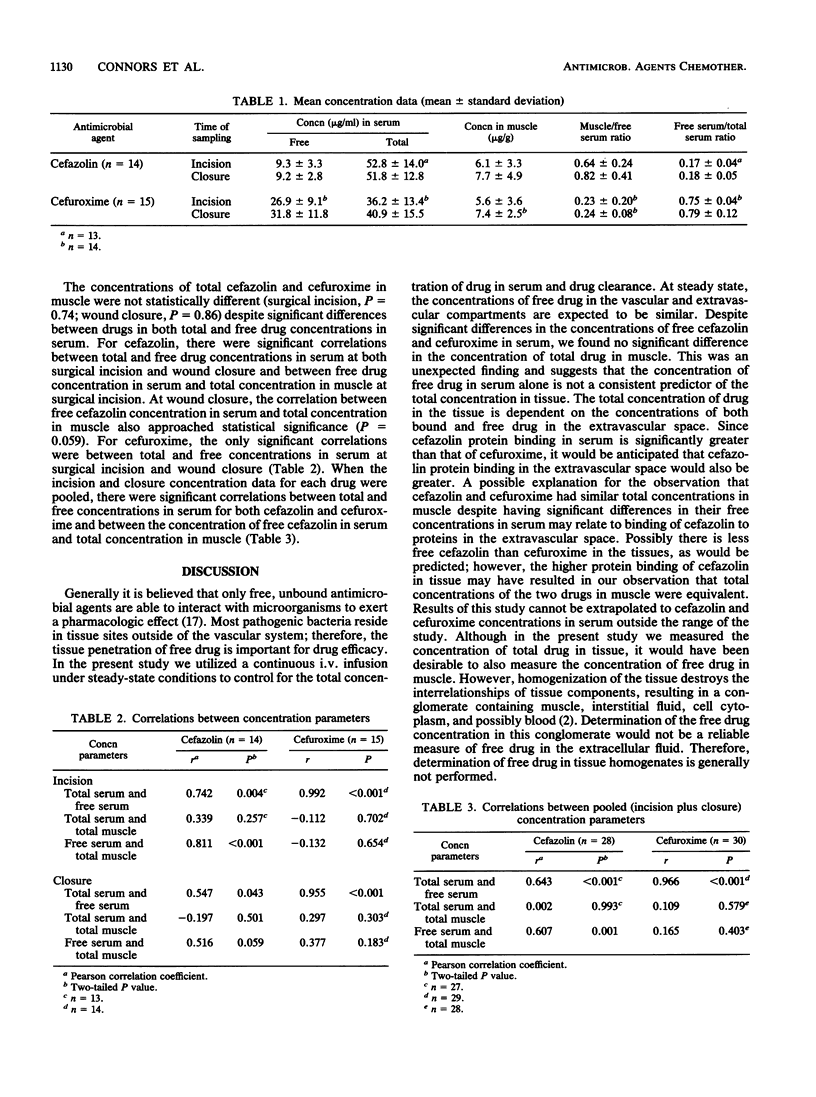
A continuous intravenous infusion was used to assess the tissue penetration of cefazolin (14 subjects) and cefuroxime (15 subjects) in orthopedic surgery patients. Subjects were randomly assigned to receive a continuous intravenous infusion of cefazolin (mean, 178.6 mg/h) or cefuroxime (mean, 330.0 mg/h) at a rate estimated to achieve a target steady-state total concentration of 50 micrograms/ml in serum. The infusion was initiated 12 to 14 h before surgery, and blood and muscle tissue samples were collected intraoperatively at the times of incision and wound closure. Although there was a significant difference between the free concentrations of cefazolin (at incision, 9.3 micrograms/ml; at closure, 9.2 micrograms/ml) and cefuroxime in serum (at incision, 26.9 micrograms/ml; at closure, 31.8 micrograms/ml), there was no difference in the total concentrations in muscle at either surgical incision (cefazolin, 6.1 micrograms/g; cefuroxime, 5.6 micrograms/g) or wound closure (cefazolin, 7.7 micrograms/g; cefuroxime, 7.4 micrograms/g). There was a significant correlation between the pooled free serum and total muscle concentrations for cefazolin (P = 0.001); however, there was no correlation between these variables with the pooled cefuroxime data (P = 0.403). These findings indicate that the free drug concentration in serum alone is not consistently predictive of the total concentration of cephalosporin in muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergan T. Pharmacokinetics of tissue penetration of antibiotics. Rev Infect Dis. 1981 Jan-Feb;3(1):45–66. doi: 10.1093/clinids/3.1.45. [DOI] [PubMed] [Google Scholar]
- Bundtzen R. W., Toothaker R. D., Nielson O. S., Madsen P. O., Welling P. G., Craig W. A. Pharmacokinetics of cefuroxime in normal and impaired renal function: comparison of high-pressure liquid chromatography and microbiological assays. Antimicrob Agents Chemother. 1981 Mar;19(3):443–449. doi: 10.1128/aac.19.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Cousins M. J., Greenstein L. R., Hitt B. A., Mazze R. I. Metabolism and renal effects of enflurane in man. Anesthesiology. 1976 Jan;44(1):44–53. doi: 10.1097/00000542-197601000-00009. [DOI] [PubMed] [Google Scholar]
- Davies A. J., Lockley R. M., Jones A., el-Safty M., Clothier J. C. Comparative pharmacokinetics of cefamandole, cefuroxime and cephradine during total hip replacement. J Antimicrob Chemother. 1986 May;17(5):637–640. doi: 10.1093/jac/17.5.637. [DOI] [PubMed] [Google Scholar]
- DiPiro J. T., Vallner J. J., Bowden T. A., Jr, Clark B. A., Sisley J. F. Intraoperative serum and tissue activity of cefazolin and cefoxitin. Arch Surg. 1985 Jul;120(7):829–832. doi: 10.1001/archsurg.1985.01390310067015. [DOI] [PubMed] [Google Scholar]
- Gower P. E., Dash C. H. The pharmacokinetics of cefuroxime after intravenous injection. Eur J Clin Pharmacol. 1977 Nov 14;12(3):221–227. doi: 10.1007/BF00609865. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Gavan T. L., Gerlach E. H., Barry A. L., Thornsberry C. Cefuroxime, a new parenteral cephalosporin: collaborative in vitro susceptibility comparison with cephalothin against 5,887 clinical bacterial isolates. Antimicrob Agents Chemother. 1977 Jul;12(1):47–50. doi: 10.1128/aac.12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., DiPiro J. T., Nix D. E., Bhatti N. A. Cephalosporins for prophylaxis in operative repair of femoral fractures. Levels in serum, muscle, and hematoma. J Bone Joint Surg Am. 1985 Jul;67(6):921–924. [PubMed] [Google Scholar]
- Järnberg P. O., Santesson J., Eklund J. Renal function during neurolept anaesthesia. Acta Anaesthesiol Scand. 1978;22(2):167–172. doi: 10.1111/j.1399-6576.1978.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Knothe H. Microbiological activity of cefazedone as compared to cefazolin and cephalothin. Arzneimittelforschung. 1979;29(2A):378–381. [PubMed] [Google Scholar]
- LeBel M., Spino M. Pulse dosing versus continuous infusion of antibiotics. Pharmacokinetic-pharmacodynamic considerations. Clin Pharmacokinet. 1988 Feb;14(2):71–95. doi: 10.2165/00003088-198814020-00002. [DOI] [PubMed] [Google Scholar]
- Mazze R. I., Cousins M. J., Barr G. A. Renal effects and metabolism of isoflurane in man. Anesthesiology. 1974 Jun;40(6):536–542. doi: 10.1097/00000542-197406000-00006. [DOI] [PubMed] [Google Scholar]
- Ryan D. M., Cars O., Hoffstedt B. The use of antibiotic serum levels to predict concentrations in tissues. Scand J Infect Dis. 1986;18(5):381–388. doi: 10.3109/00365548609032352. [DOI] [PubMed] [Google Scholar]
- Wise R. The clinical relevance of protein binding and tissue concentrations in antimicrobial therapy. Clin Pharmacokinet. 1986 Nov-Dec;11(6):470–482. doi: 10.2165/00003088-198611060-00004. [DOI] [PubMed] [Google Scholar]


