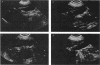
Abstract
Biliary pseudolithiasis has been reported in patients who received ceftriaxone therapy. To examine this phenomenon further, serial gallbladder sonograms were evaluated in 44 adult patients who received intravenous ceftriaxone at 2 g or a placebo daily for 14 days in a double-blind controlled study. Ultrasound examinations of gallbladders were performed on days 1 and 14 of therapy and 2 weeks posttherapy if abnormalities were observed on day 14. Eight patients were unevaluable because of abnormal base-line gallbladder sonograms. Thirty-six patients (ceftriaxone, n = 28; placebo, n = 8) demonstrated normal baseline gallbladder sonograms and were evaluated for the development of change. A total of 6 of 28 (21.4%) ceftriaxone-treated patients and 1 of 8 (12.5%) patients who received the placebo demonstrated abnormal gallbladder sonograms on day 14 (P = 0.491). Four of the six ceftriaxone-treated patients demonstrating abnormal sonograms were clinically asymptomatic, while two patients reported vomiting. The abnormal sonograms of gallbladders of patients treated with ceftriaxone returned to normal between 9 and 26 days posttherapy. These data suggest an association between ceftriaxone treatment and the development of gallbladder abnormalities on ultrasound examination which resolve spontaneously on discontinuation of ceftriaxone therapy.
Full text
PDF
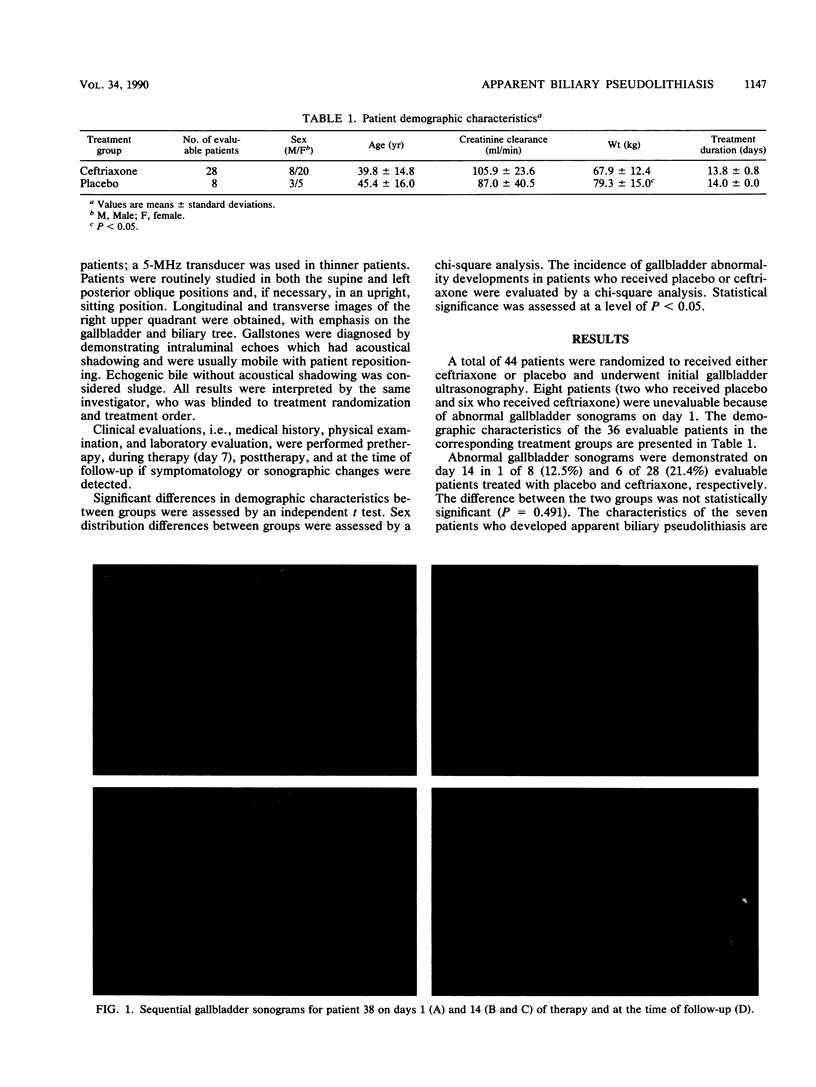
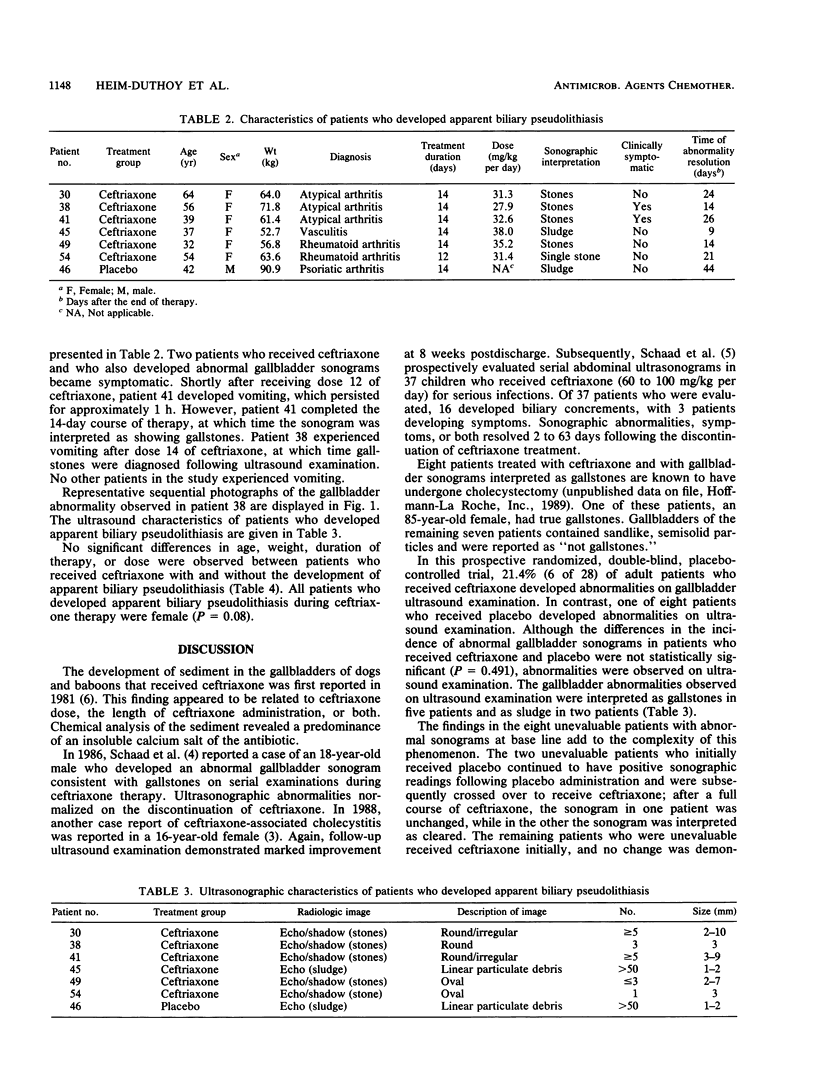
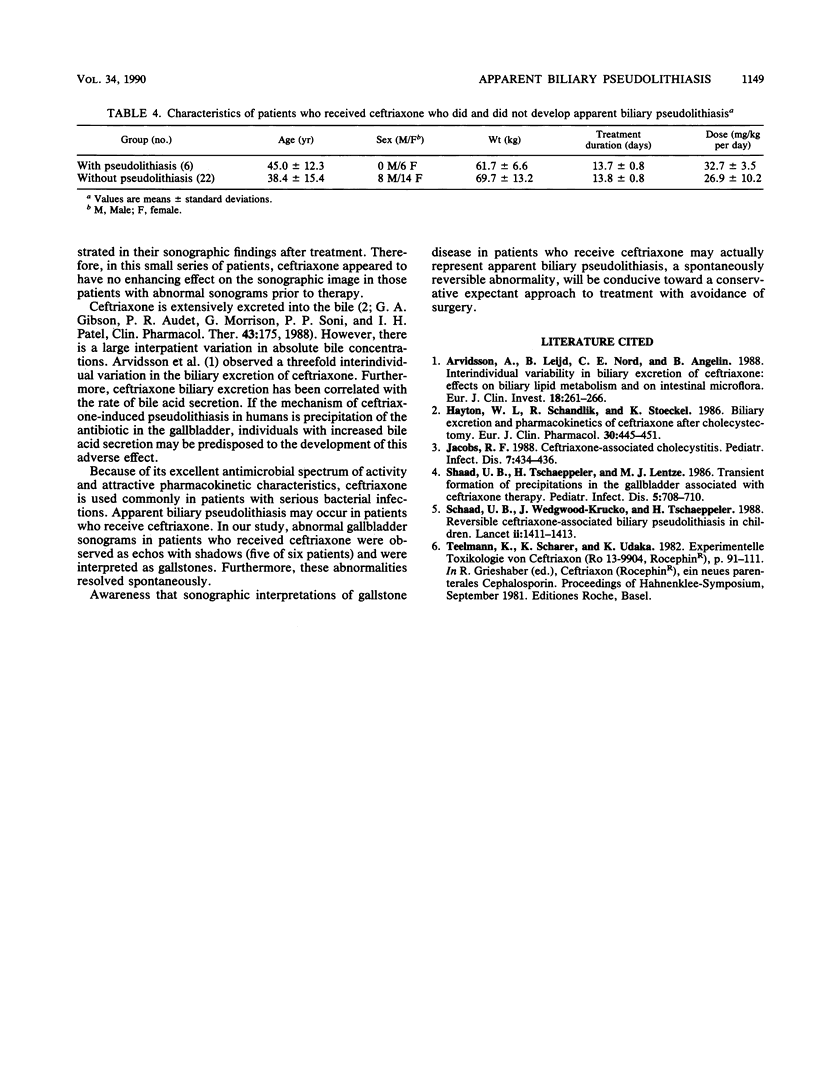
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson A., Leijd B., Nord C. E., Angelin B. Interindividual variability in biliary excretion of ceftriaxone: effects on biliary lipid metabolism and on intestinal microflora. Eur J Clin Invest. 1988 Jun;18(3):261–266. doi: 10.1111/j.1365-2362.1988.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Hayton W. L., Schandlik R., Stoeckel K. Biliary excretion and pharmacokinetics of ceftriaxone after cholecystectomy. Eur J Clin Pharmacol. 1986;30(4):445–451. doi: 10.1007/BF00607958. [DOI] [PubMed] [Google Scholar]
- Jacobs R. F. Ceftriaxone-associated cholecystitis. Pediatr Infect Dis J. 1988 Jun;7(6):434–436. doi: 10.1097/00006454-198806000-00018. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Tschäppeler H., Lentze M. J. Transient formation of precipitations in the gallbladder associated with ceftriaxone therapy. Pediatr Infect Dis. 1986 Nov-Dec;5(6):708–710. doi: 10.1097/00006454-198611000-00026. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Wedgwood-Krucko J., Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet. 1988 Dec 17;2(8625):1411–1413. doi: 10.1016/s0140-6736(88)90596-x. [DOI] [PubMed] [Google Scholar]