Abstract
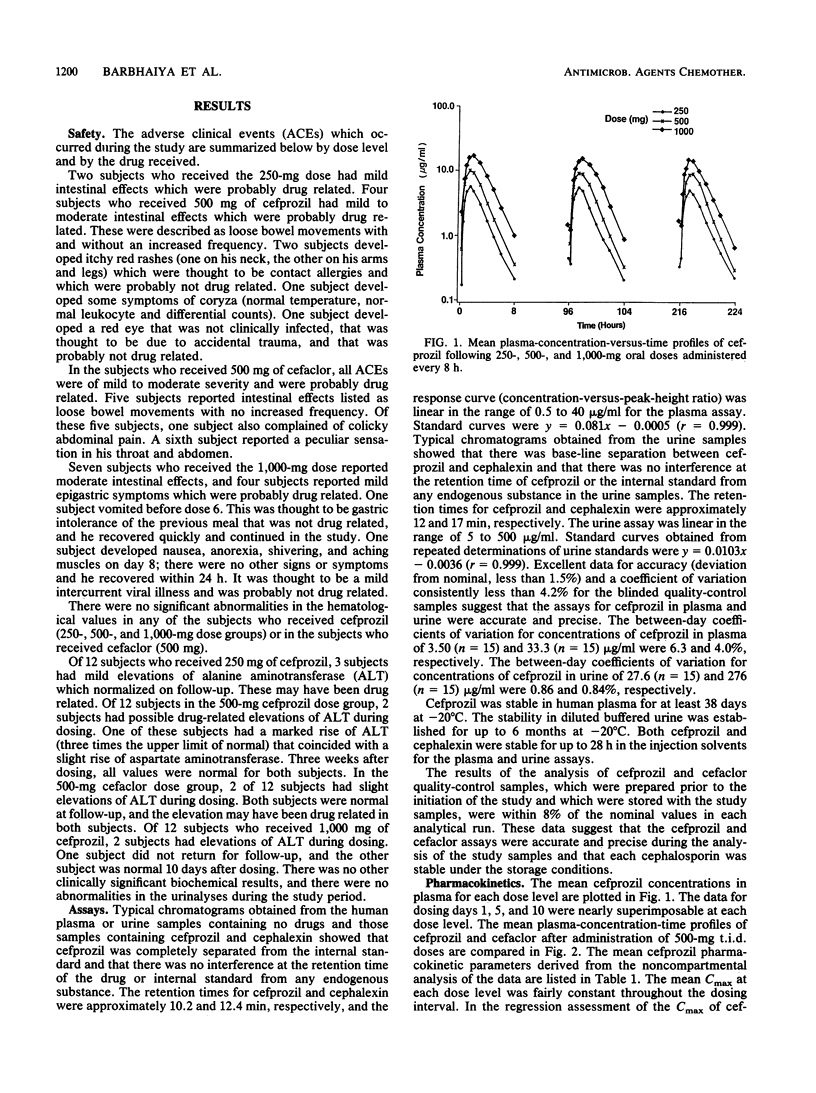
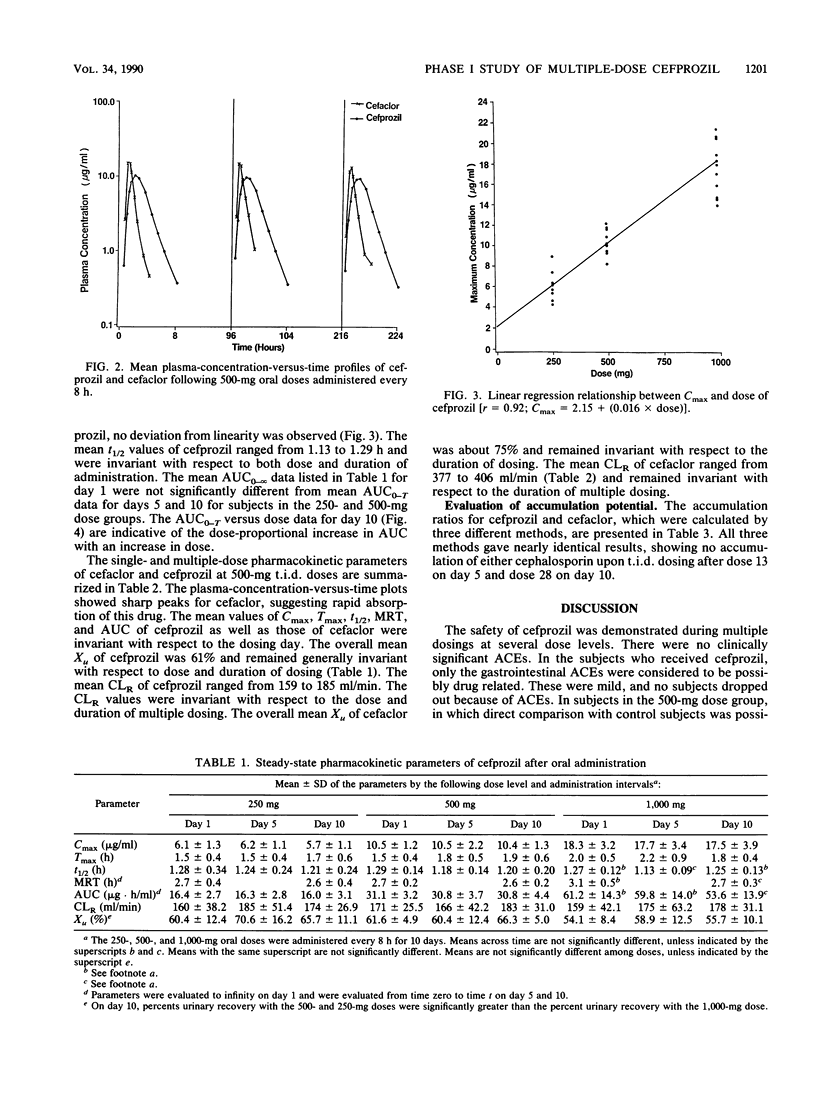
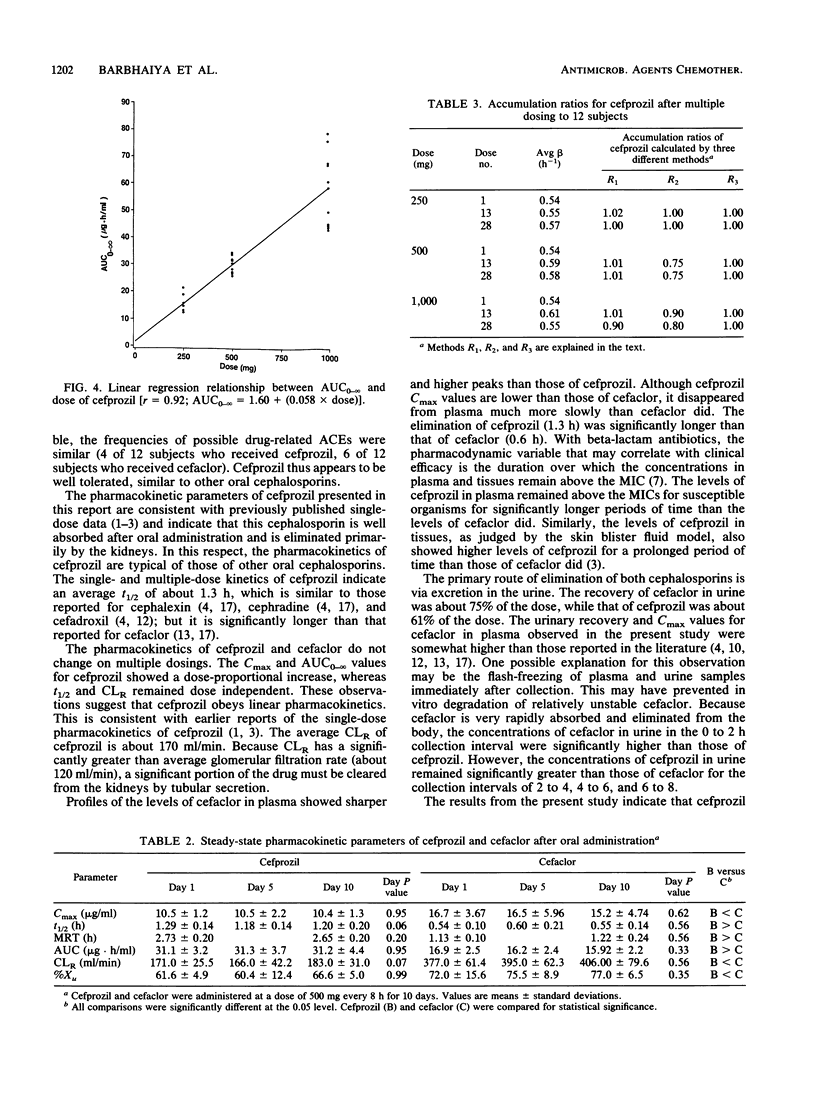
The objectives of this study were to assess the safety and tolerance of cefprozil, to characterize the pharmacokinetics of cefprozil after administration of multiple doses of the drug, and to compare these pharmacokinetic parameters with those obtained with cefaclor. The volunteers received 28 doses of 250, 500, or 1,000 mg of cefprozil or 500 mg of cefaclor every 8 h for 10 days. Serial blood samples and the total volume of urine voided by each individual were collected for pharmacokinetic evaluation on days 1, 5, and 10. Both cephalosporins were well tolerated after multiple oral dosing. The peak levels in plasma (Cmax) of cefprozil ranged from 5.7 to 18.3 micrograms/ml after oral administration of 250- to 1,000-mg doses. The regression analysis of Cmax on cefprozil dose showed a dose-linear response. The mean Cmax of cefaclor ranged from 15.2 to 16.7 micrograms/ml and did not change significantly on multiple dosing. The overall mean terminal half-life of cefprozil was 1.2 h and was invariant with respect to dose or duration of dosing. The area under the plasma-concentration-versus-time curve from 0 h to infinity (AUC0-infinity) of cefprozil increased in a dose-proportional manner with an increase in dose. The overall urinary recovery (61% of dose) and renal clearance values of cefprozil were generally invariant with respect to dose and duration of dosing. While cefprozil was apparently absorbed less rapidly and achieved lower Cmax values than cefaclor, the AUC0-infinity of cefprozil was nearly twofold greater than that of cefaclor. The half-life of cefprozil was also twofold longer than that observed for cefaclor. Although the urinary recovery of cefaclor (75% of dose) was significantly higher than that of cefprozil (61% of dose), the concentrations of cefprozil in urine remained significantly higher than those of cefaclor from 2 to 8 h postdosing. If the therapeutic concept is maintained that levels of beta-lactam antibiotics in plasma should exceed the MIC for the offending organisms over a period that approximates the dosing interval, then cefprozil would appear to be suitable for twice-daily administration, whereas cefaclor should probably be administered three or even four times a day.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Gleason C. R., Shyu W. C., Wilber R. B., Martin R. R., Pittman K. A. Phase I study of single-dose BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1990 Feb;34(2):202–205. doi: 10.1128/aac.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Pittman K. A. Comparison of the effects of food on the pharmacokinetics of cefprozil and cefaclor. Antimicrob Agents Chemother. 1990 Jun;34(6):1210–1213. doi: 10.1128/aac.34.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Wilber R. B., Pittman K. A. Comparison of cefprozil and cefaclor pharmacokinetics and tissue penetration. Antimicrob Agents Chemother. 1990 Jun;34(6):1204–1209. doi: 10.1128/aac.34.6.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Comparative antibacterial activity of a new oral cephalosporin, BMY-28100. Antimicrob Agents Chemother. 1987 Mar;31(3):480–483. doi: 10.1128/aac.31.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn W. A. Estimating the accumulation of drugs. J Pharm Sci. 1983 Jul;72(7):833–834. doi: 10.1002/jps.2600720734. [DOI] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Reiszner E., Wennersten C., Moellering R. C., Jr In vitro activity of BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Apr;31(4):653–656. doi: 10.1128/aac.31.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges G. R., Liu C., Hinthorn D. R., Harms J. L., Dworzack D. L. Pharmacological evaluation of cefaclor in volunteers. Antimicrob Agents Chemother. 1978 Sep;14(3):454–456. doi: 10.1128/aac.14.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner F., Pursiano T. A., Buck R. E., Tsai Y. H., Chisholm D. R., Misiek M., Desiderio J. V., Kessler R. E. BMY 28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Feb;31(2):238–243. doi: 10.1128/aac.31.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Stahlmann R., Koeppe P. Comparative pharmacokinetics of cephalexin, cefaclor, cefadroxil, and CGP 9000. Antimicrob Agents Chemother. 1979 Jul;16(1):1–6. doi: 10.1128/aac.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers B. R., Hirschman S. Z., Wormser G., Gartenberg G., Srulevitch E. Pharmacologic studies with cefaclor, a new oral cephalosporin. J Clin Pharmacol. 1978 Apr;18(4):174–179. doi: 10.1002/j.1552-4604.1978.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Nahata M. C. Determination of cefaclor by high-performance liquid chromatography. J Chromatogr. 1982 Mar 12;228:429–433. doi: 10.1016/s0378-4347(00)80468-5. [DOI] [PubMed] [Google Scholar]
- Pfeffer M. Estimation of mean residence time from data obtained when multiple-dosing steady state has been reached. J Pharm Sci. 1984 Jun;73(6):854–856. doi: 10.1002/jps.2600730645. [DOI] [PubMed] [Google Scholar]
- Scribner R. K., Marks M. I., Finkhouse B. D. In vitro activity of BMY-28100 against common isolates from pediatric infections. Antimicrob Agents Chemother. 1987 Apr;31(4):630–631. doi: 10.1128/aac.31.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling P. G., Dean S., Selen A., Kendall M. J., Wise R. The pharmacokinetics of the oral cephalosporins cefaclor, cephradine and cephalexin. Int J Clin Pharmacol Biopharm. 1979 Sep;17(9):397–400. [PubMed] [Google Scholar]


