Abstract
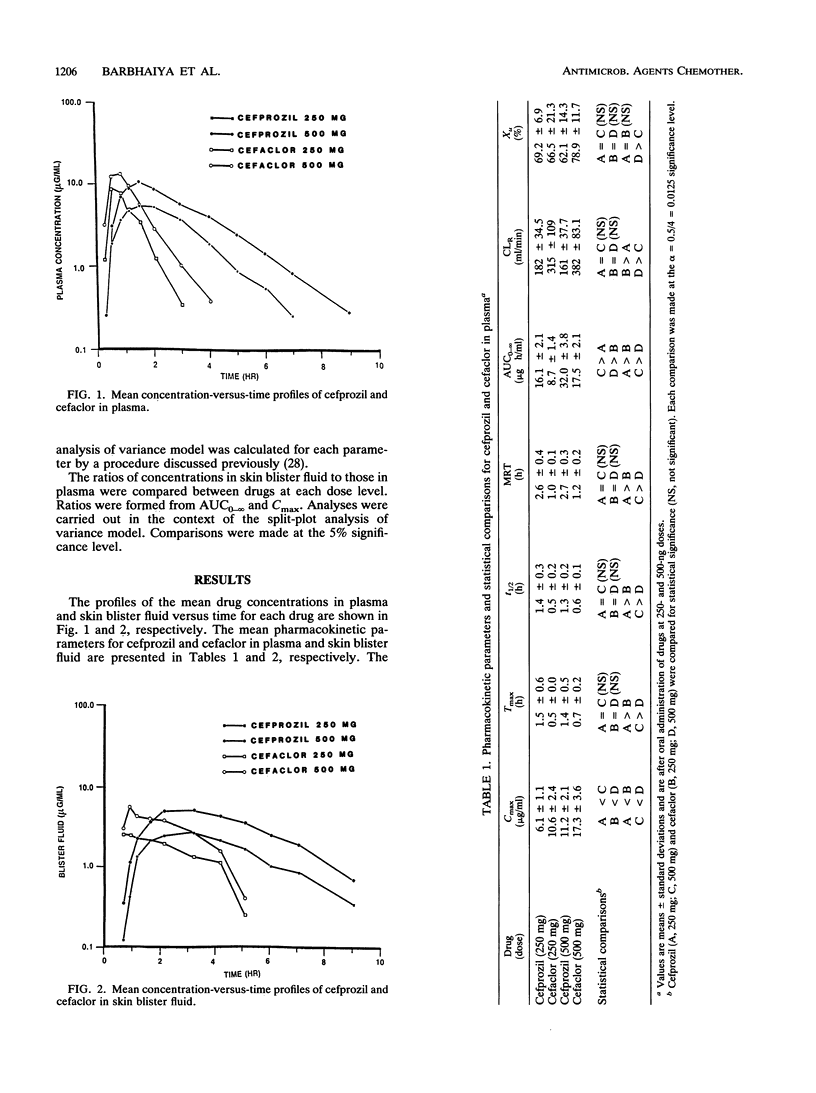
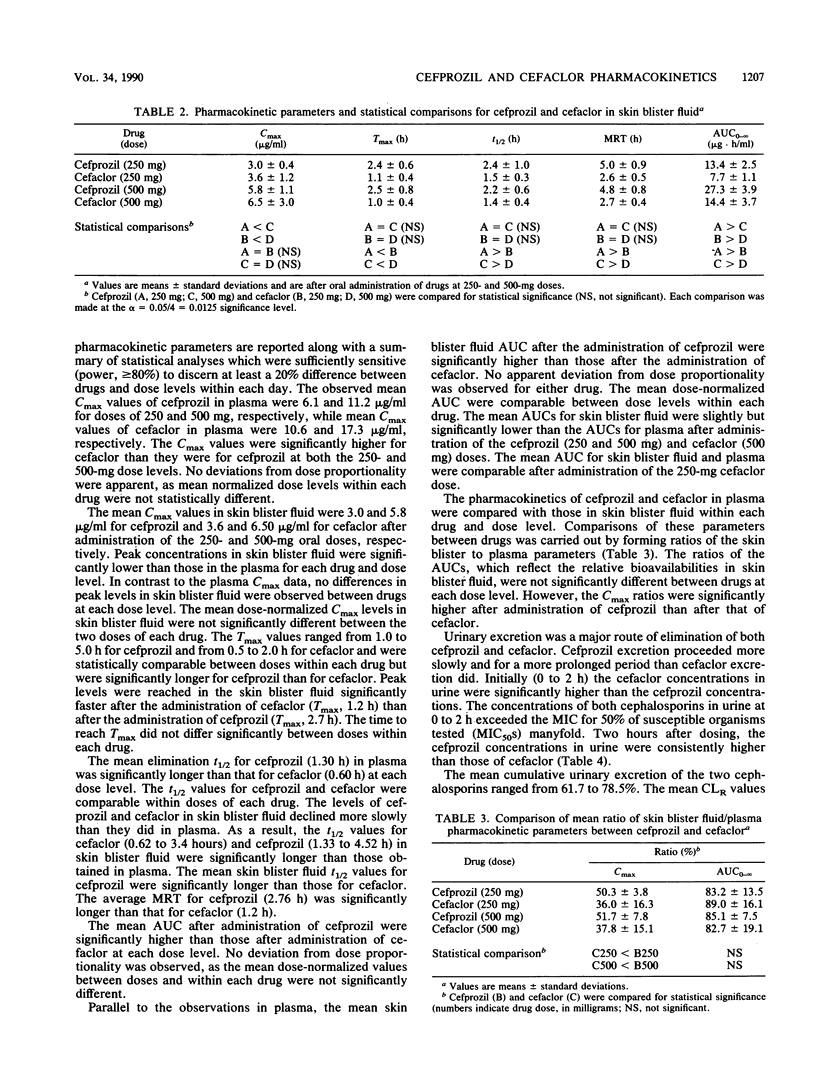
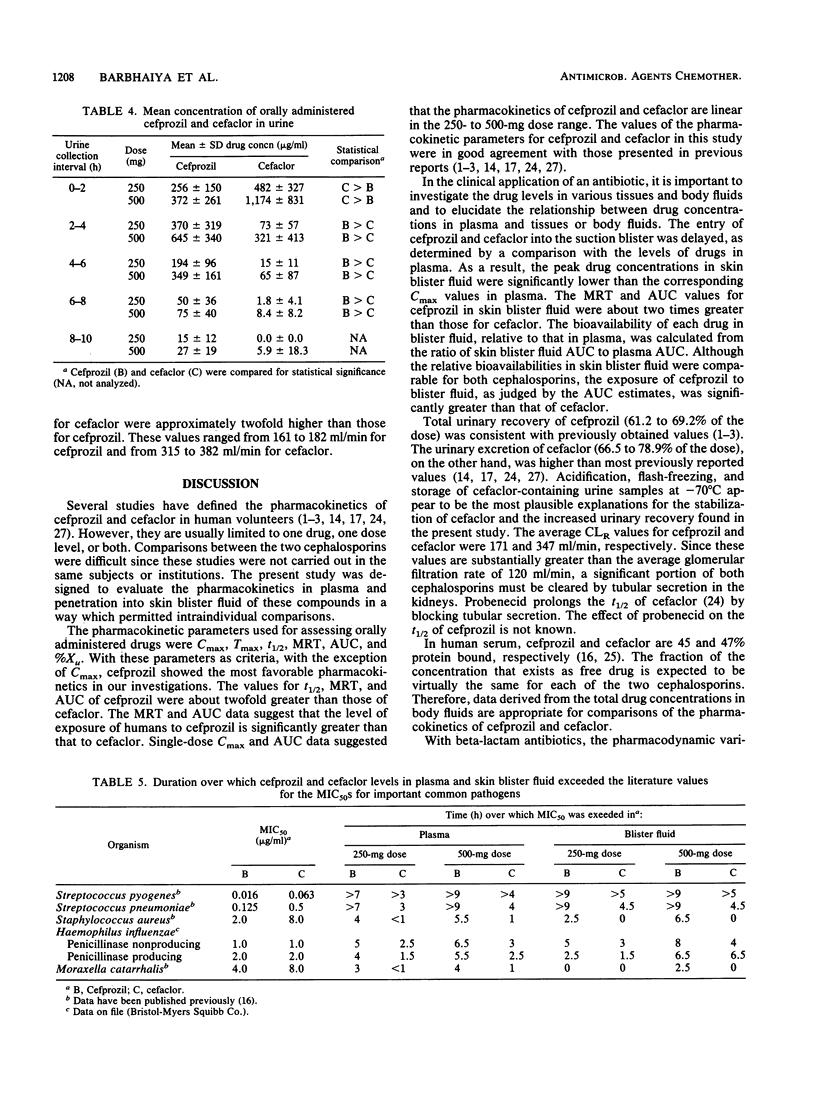
The pharmacokinetics and tissue penetration, as judged by skin blister fluid, of cefprozil and cefaclor were examined in 12 healthy male volunteers. Doses of 250 and 500 mg of each drug were given to fasting subjects in a crossover fashion. Serially obtained plasma, skin blister fluid, and urine samples were analyzed for cefprozil or cefaclor by validated high-pressure liquid chromatographic methods. After oral administration of 250 and 500 mg of cefprozil, mean concentrations in plasma rose to peak levels (Cmax) of 6.1 and 11.2 micrograms/ml, respectively, and those of cefaclor were 10.6 and 17.3 micrograms/ml, respectively. The elimination half-life of cefprozil (1.3 h) was significantly longer than that of cefaclor (0.6 h), and as a result, the area under the curve for cefprozil was about two times greater than that for cefaclor. Both cephalosporins were primarily excreted unchanged in urine. The mean skin blister Cmax values were 3.0 and 5.8 micrograms/ml for cefprozil and 3.6 and 6.5 micrograms/ml for cefaclor after the 250- and 500-mg oral doses, respectively. The mean Cmax values in skin blister fluid for both cephalosporins were comparable and were significantly lower than the corresponding Cmax values in plasma. However, the levels of cefprozil and cefaclor in skin blister fluid declined more slowly than they did in plasma. The skin blister fluid half-life estimates for cefprozil were significantly longer than they were for cefaclor. Parallel to the observation in plasma, the mean skin blister fluid areas under the curve for cefprozil were significantly higher than they were for cefaclor. The plasma and skin blister fluid pharmacokinetic analyses suggest that the exposure of humans to cefprozil is significantly greater than that to cefaclor at the same dose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Sykes N. S., Mitchell M. M., Fisher M. W. Concentration of selected intravenously administered antibiotics in experimental surgical wounds. J Trauma. 1973 May;13(5):423–434. doi: 10.1097/00005373-197305000-00004. [DOI] [PubMed] [Google Scholar]
- Barbhaiya R. H., Gleason C. R., Shyu W. C., Wilber R. B., Martin R. R., Pittman K. A. Phase I study of single-dose BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1990 Feb;34(2):202–205. doi: 10.1128/aac.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Pittman K. A. Comparison of the effects of food on the pharmacokinetics of cefprozil and cefaclor. Antimicrob Agents Chemother. 1990 Jun;34(6):1210–1213. doi: 10.1128/aac.34.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Wilber R. B., Martin R. R., Pittman K. A. Phase I study of multiple-dose cefprozil and comparison with cefaclor. Antimicrob Agents Chemother. 1990 Jun;34(6):1198–1203. doi: 10.1128/aac.34.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Comparative antibacterial activity of a new oral cephalosporin, BMY-28100. Antimicrob Agents Chemother. 1987 Mar;31(3):480–483. doi: 10.1128/aac.31.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Reiszner E., Wennersten C., Moellering R. C., Jr In vitro activity of BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Apr;31(4):653–656. doi: 10.1128/aac.31.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglesong M. A., Lamb J. W., Dietz J. V. Stability and blood level determinations of cefaclor, a new oral cephalosporin antibiotic. Antimicrob Agents Chemother. 1978 Jan;13(1):49–52. doi: 10.1128/aac.13.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frongillo R. F., Galuppo L., Moretti A. Suction skin blister, skin window, and skin chamber techniques to determine extravascular passage of cefotaxime in humans. Antimicrob Agents Chemother. 1981 Jan;19(1):22–28. doi: 10.1128/aac.19.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Hall W. H., Schierl E. A., Manion R. E. Cephalosporin and aminoglycoside concentrations in peritoneal capsular fluid in rabbits. Antimicrob Agents Chemother. 1976 Dec;10(6):902–911. doi: 10.1128/aac.10.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges G. R., Liu C., Hinthorn D. R., Harms J. L., Dworzack D. L. Pharmacological evaluation of cefaclor in volunteers. Antimicrob Agents Chemother. 1978 Sep;14(3):454–456. doi: 10.1128/aac.14.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiistala U., Mustakallio K. K. Dermo-epidermal separation with suction. Electron microscopic and histochemical study of initial events of blistering on human skin. J Invest Dermatol. 1967 May;48(5):466–477. [PubMed] [Google Scholar]
- Leitner F., Pursiano T. A., Buck R. E., Tsai Y. H., Chisholm D. R., Misiek M., Desiderio J. V., Kessler R. E. BMY 28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Feb;31(2):238–243. doi: 10.1128/aac.31.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Stahlmann R., Koeppe P. Comparative pharmacokinetics of cephalexin, cefaclor, cefadroxil, and CGP 9000. Antimicrob Agents Chemother. 1979 Jul;16(1):1–6. doi: 10.1128/aac.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahata M. C. Determination of cefaclor by high-performance liquid chromatography. J Chromatogr. 1982 Mar 12;228:429–433. doi: 10.1016/s0378-4347(00)80468-5. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980 Oct;8(5):509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]
- Ryan D. M., Hodges B., Spencer G. R., Harding S. M. Simultaneous comparison of three methods for assessing ceftazidime penetration into extravascular fluid. Antimicrob Agents Chemother. 1982 Dec;22(6):995–998. doi: 10.1128/aac.22.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. M. Implanted tissue-cages: a critical evaluation of their relevance in measuring tissue concentrations of antibiotics. Scand J Infect Dis Suppl. 1978;(13):58–62. [PubMed] [Google Scholar]
- Santoro J., Agarwal B. N., Martinelli R., Wenger N., Levison M. E. Pharmacology of cefaclor in normal volunteers and patients with renal failure. Antimicrob Agents Chemother. 1978 Jun;13(6):951–954. doi: 10.1128/aac.13.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner A., Hellum K. B., Digranes A., Bergman I. Transfer of penicillin G and ampicillin into human skin blisters induced by suction. Scand J Infect Dis Suppl. 1978;(14):233–237. [PubMed] [Google Scholar]
- Shyu W. C., Quintiliani R., Nightingale C. H. An improved method to determine interstitial fluid pharmacokinetics. J Infect Dis. 1985 Dec;152(6):1328–1331. doi: 10.1093/infdis/152.6.1328. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Barza M. In vitro activity and serum protein-binding of cefaclor. J Antimicrob Chemother. 1979 Mar;5(2):159–165. doi: 10.1093/jac/5.2.159. [DOI] [PubMed] [Google Scholar]
- Waterman N. G., Kastan L. B. Interstitial fluid and serum antibiotic concentrations. Arch Surg. 1972 Aug;105(2):192–196. doi: 10.1001/archsurg.1972.04180080046008. [DOI] [PubMed] [Google Scholar]
- Welling P. G., Dean S., Selen A., Kendall M. J., Wise R. The pharmacokinetics of the oral cephalosporins cefaclor, cephradine and cephalexin. Int J Clin Pharmacol Biopharm. 1979 Sep;17(9):397–400. [PubMed] [Google Scholar]


