Abstract
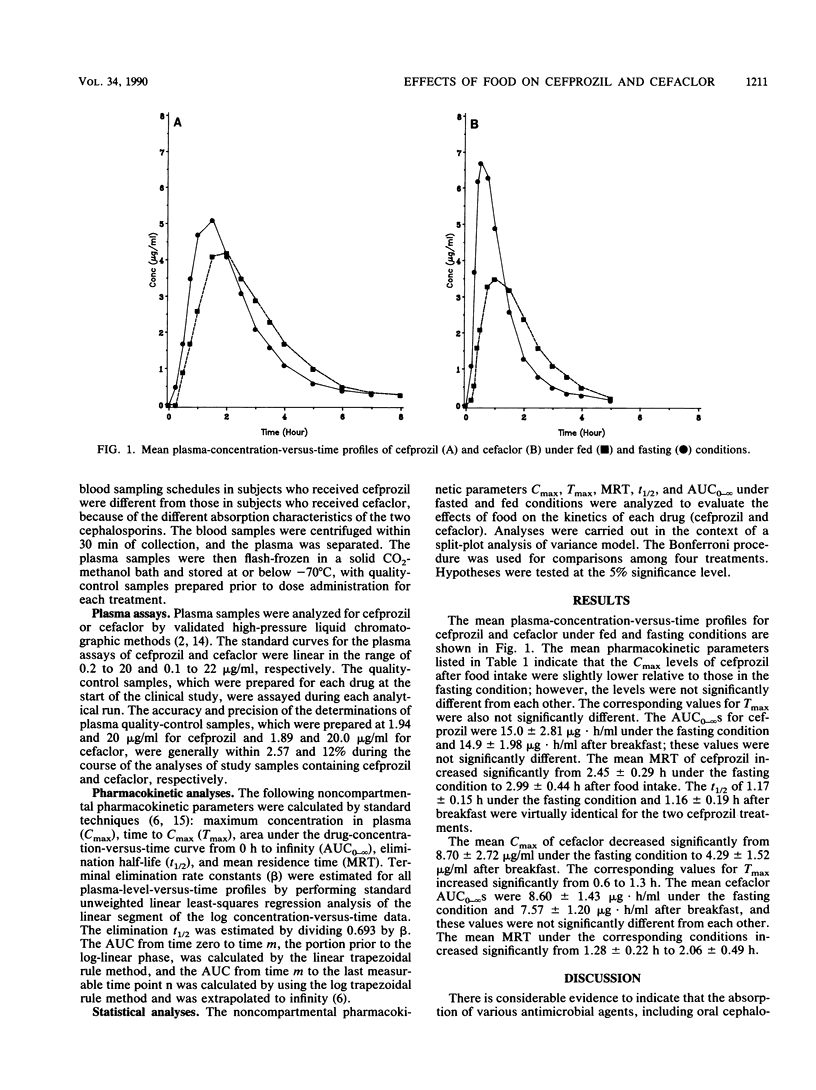
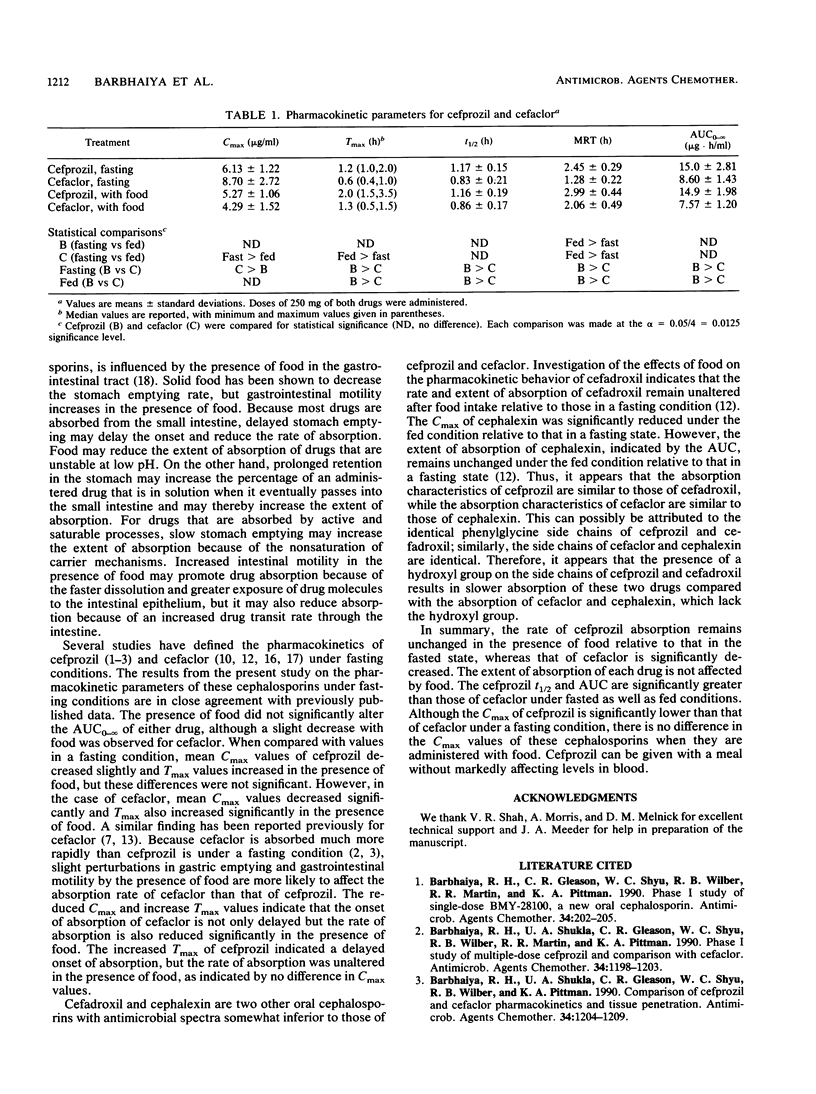
The objective of this study was to assess the effects of food on the pharmacokinetics of cefprozil and cefaclor. A group of 12 healthy male volunteers received a single 250-mg dose of cefprozil or cefaclor under fasting conditions as well as after the intake of food. There was a 1-week washout period between each treatment. Serial blood samples were collected and assayed for cefprozil or cefaclor by specific high-pressure liquid chromatographic methods. The mean +/- standard deviation peak concentration (Cmax) of cefprozil in plasma was 6.13 +/- 1.22 micrograms/ml under the fasting condition and 5.27 +/- 1.06 micrograms/ml after breakfast, and these values were not significantly different from each other. The corresponding median time to reach Cmax was prolonged after food intake, but this difference was not significant. The mean Cmax values of cefaclor decreased significantly from 8.70 +/- 2.72 micrograms/ml under the fasting condition to 4.29 +/- 1.52 micrograms/ml after breakfast, and the corresponding median times to reach Cmax were significantly prolonged. The mean half-lives of cefprozil and cefaclor were nearly identical for the two treatments, suggesting that the elimination kinetics of these cephalosporins remained unaltered when the drugs were administered with food. The area under the plasma-concentration-versus-time curves for fasted and fed conditions were not significantly different for both drugs. The results of this study indicate that the extent of absorption and rate of elimination of both cephalosporins remain unaltered in the presence of food. However, the absorption rate of cefaclor is significantly reduced in the presence of food, while that of cefprozil remains unaltered. As a result, the Cmax of cefaclor is significantly reduced in the presence of food, whereas that of cefprozil is not significantly affected. Cefprozil can be administered with a meal without markedly affecting levels in blood.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Gleason C. R., Shyu W. C., Wilber R. B., Martin R. R., Pittman K. A. Phase I study of single-dose BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1990 Feb;34(2):202–205. doi: 10.1128/aac.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Wilber R. B., Martin R. R., Pittman K. A. Phase I study of multiple-dose cefprozil and comparison with cefaclor. Antimicrob Agents Chemother. 1990 Jun;34(6):1198–1203. doi: 10.1128/aac.34.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Wilber R. B., Pittman K. A. Comparison of cefprozil and cefaclor pharmacokinetics and tissue penetration. Antimicrob Agents Chemother. 1990 Jun;34(6):1204–1209. doi: 10.1128/aac.34.6.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Reiszner E., Wennersten C., Moellering R. C., Jr In vitro activity of BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Apr;31(4):653–656. doi: 10.1128/aac.31.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Reiszner E., Wennersten C., Moellering R. C., Jr In vitro activity of BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Apr;31(4):653–656. doi: 10.1128/aac.31.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynne A., Goulbourn R. A., Ryden R. A human pharmacology study of cefaclor. J Antimicrob Chemother. 1978 Jul;4(4):343–348. doi: 10.1093/jac/4.4.343. [DOI] [PubMed] [Google Scholar]
- Haginaka J., Yamaoka K., Nakagawa T., Nishimura Y., Uno T. Evaluation of effect of food ingestion on bioavailability of cephalexin by moment analysis. Chem Pharm Bull (Tokyo) 1979 Dec;27(12):3156–3159. doi: 10.1248/cpb.27.3156. [DOI] [PubMed] [Google Scholar]
- Harvengt C., De Schepper P., Lamy F., Hansen J. Cephradine absorption and excretion in fasting and nonfasting volunteers. J Clin Pharmacol New Drugs. 1973 Jan;13(1):36–40. doi: 10.1002/j.1552-4604.1973.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Hodges G. R., Liu C., Hinthorn D. R., Harms J. L., Dworzack D. L. Pharmacological evaluation of cefaclor in volunteers. Antimicrob Agents Chemother. 1978 Sep;14(3):454–456. doi: 10.1128/aac.14.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner F., Pursiano T. A., Buck R. E., Tsai Y. H., Chisholm D. R., Misiek M., Desiderio J. V., Kessler R. E. BMY 28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Feb;31(2):238–243. doi: 10.1128/aac.31.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Stahlmann R., Koeppe P. Comparative pharmacokinetics of cephalexin, cefaclor, cefadroxil, and CGP 9000. Antimicrob Agents Chemother. 1979 Jul;16(1):1–6. doi: 10.1128/aac.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Ginsburg C. M., Clahsen J. C., Thomas M. L. Pharmacologic evaluation of orally administered antibiotics in infants and children: effect of feeding on bioavailability. Pediatrics. 1978 Nov;62(5):738–743. [PubMed] [Google Scholar]
- Nahata M. C. Determination of cefaclor by high-performance liquid chromatography. J Chromatogr. 1982 Mar 12;228:429–433. doi: 10.1016/s0378-4347(00)80468-5. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980 Oct;8(5):509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]
- Santoro J., Agarwal B. N., Martinelli R., Wenger N., Levison M. E. Pharmacology of cefaclor in normal volunteers and patients with renal failure. Antimicrob Agents Chemother. 1978 Jun;13(6):951–954. doi: 10.1128/aac.13.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling P. G., Dean S., Selen A., Kendall M. J., Wise R. The pharmacokinetics of the oral cephalosporins cefaclor, cephradine and cephalexin. Int J Clin Pharmacol Biopharm. 1979 Sep;17(9):397–400. [PubMed] [Google Scholar]
- Welling P. G., Tse F. L. The influence of food on the absorption of antimicrobial agents. J Antimicrob Chemother. 1982 Jan;9(1):7–27. doi: 10.1093/jac/9.1.7. [DOI] [PubMed] [Google Scholar]


