Abstract
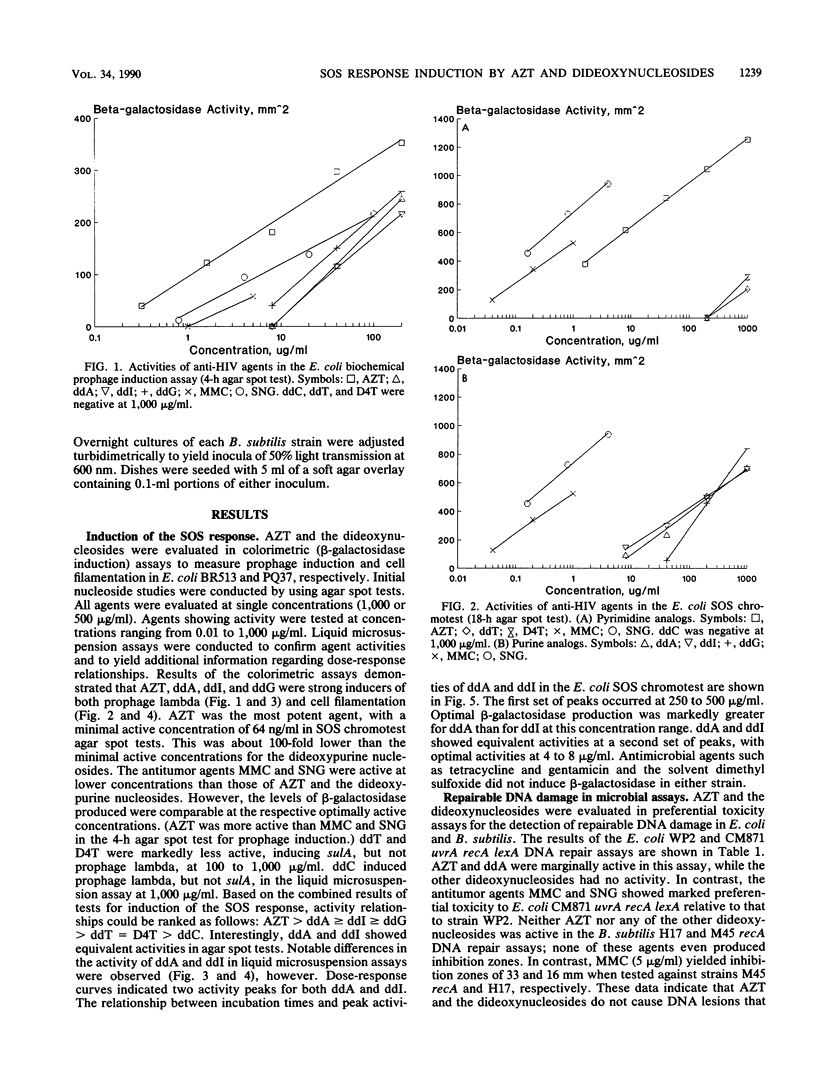
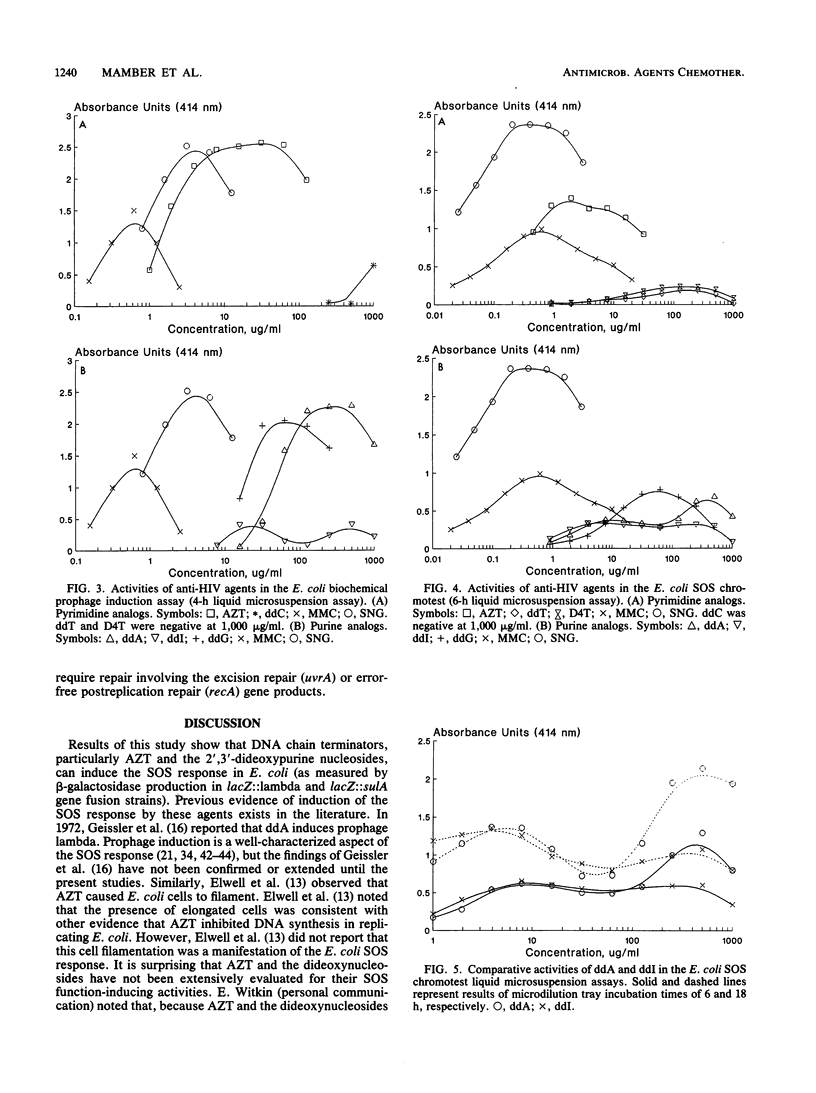
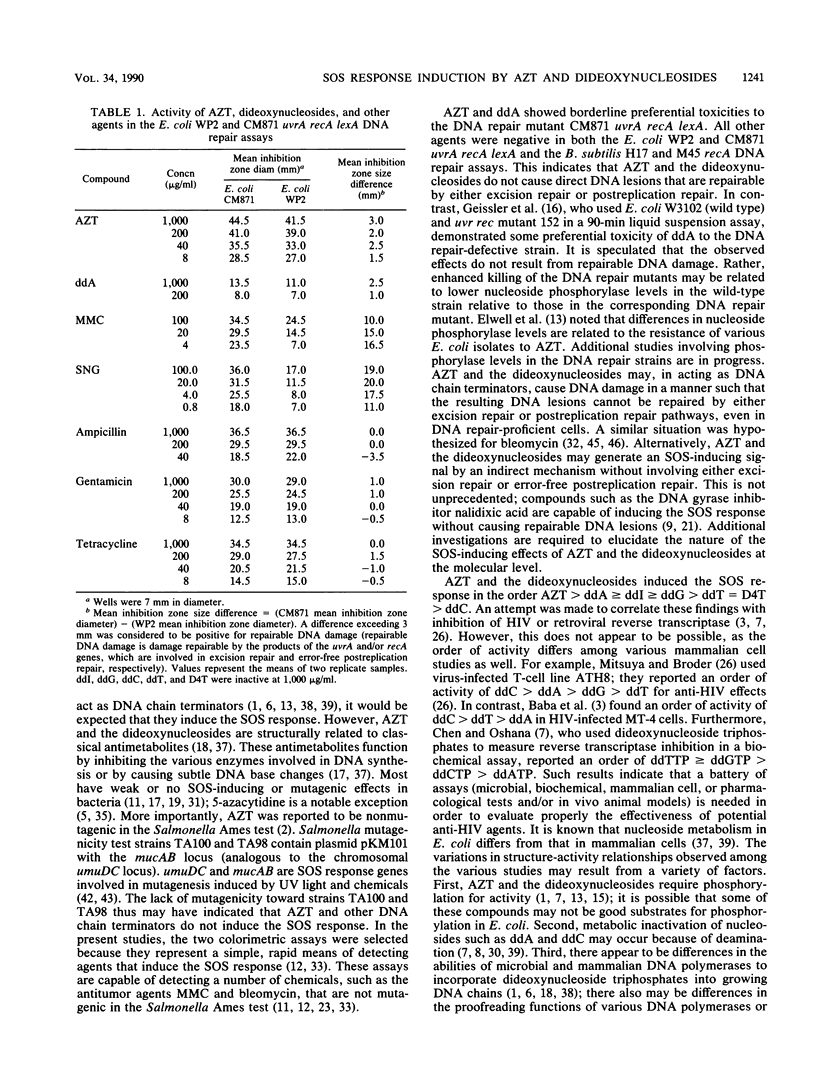
A number of nucleosides with anti-human immunodeficiency virus (HIV) activity were evaluated in two colorimetric (beta-galactosidase) assays for induction of the SOS response in Escherichia coli. 3'-Azido-3'-deoxythymidine (azidothymidine; AZT), 2',3'-dideoxyadenosine (ddA), 2',3'-dideoxyguanosine (ddG), and 2',3'-dideoxyinosine (ddI) induced cell filamentation (sulA) and prophage lambda in well-agar diffusion and liquid microsuspension assays. AZT was approximately 100 times more potent than the dideoxypurine nucleosides, inducing sulA at less than 100 ng/ml. 2',3'-Dideoxythymidine (ddT) and 2',3'-dideoxy-2',3'-didehydrothymidine (D4T) induced sulA at 100 to 1,000 micrograms/ml, while 2',3'-dideoxycytidine (ddC) weakly induced prophage lambda. Activity relationships thus were AZT greater than ddA greater than or equal to ddI greater than or equal to ddG greater than ddT = D4T greater than ddC. ddA and ddI had equivalent activities in agar diffusion assays, but different activity profiles were observed in liquid microsuspension assays. The differences may be related to drug metabolism. AZT and ddA showed marginal effects in a DNA repair (preferential toxicity) assay in which E. coli WP2 and CM871 uvrA recA lexA were used. Furthermore, none of the agents was able to preferentially inhibit Bacillus subtilis M45 recA relative to wild-type strain H17. These data suggest that AZT and the dideoxynucleosides do not cause DNA lesions that are repairable by excision repair and/or error-free postreplication repair processes. Rather, the SOS response appears to be induced by DNA chain termination leading to the inhibition of DNA replication. Bacterial assays for induction of the SOS response may be useful as simple, rapid prescreens for the discovery of new anti-HIV agents. Moreover, such assays may provide an additional parameter in the evaluation of agents with demonstrated activity against HIV and other retroviruses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. R., Deutscher M. P., Kornberg A., Russell A. F., Moffatt J. G. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2',3'-dideoxyribonucleotide. Biochemistry. 1969 Dec;8(12):4897–4904. doi: 10.1021/bi00840a037. [DOI] [PubMed] [Google Scholar]
- Ayers K. M. Preclinical toxicology of zidovudine. An overview. Am J Med. 1988 Aug 29;85(2A):186–188. [PubMed] [Google Scholar]
- Baba M., Pauwels R., Herdewijn P., De Clercq E., Desmyter J., Vandeputte M. Both 2',3'-dideoxythymidine and its 2',3'-unsaturated derivative (2',3'-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem Biophys Res Commun. 1987 Jan 15;142(1):128–134. doi: 10.1016/0006-291x(87)90460-8. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Kang G. J., Dalal M., Herdewijn P., De Clercq E., Broder S., Johns D. G. The anti-HTLV-III (anti-HIV) and cytotoxic activity of 2',3'-didehydro-2',3'-dideoxyribonucleosides: a comparison with their parental 2',3'-dideoxyribonucleosides. Mol Pharmacol. 1987 Jul;32(1):162–167. [PubMed] [Google Scholar]
- Barbé J., Gibert I., Guerrero R. 5-Azacytidine: survival and induction of the SOS response in Escherichia coli K-12. Mutat Res. 1986 Jul;166(1):9–16. doi: 10.1016/0167-8817(86)90035-0. [DOI] [PubMed] [Google Scholar]
- Beskid G., Eskin B., Cleeland R., Siebelist J., Cappetta A., Hill A. D., Geiger R. H. Antibacterial activity of 2',3'-dideoxyadenosine in vivo and in vitro. Antimicrob Agents Chemother. 1981 Mar;19(3):424–428. doi: 10.1128/aac.19.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Oshana S. C. Inhibition of HIV reverse transcriptase by 2',3'-dideoxynucleoside triphosphates. Biochem Pharmacol. 1987 Dec 15;36(24):4361–4362. doi: 10.1016/0006-2952(87)90685-x. [DOI] [PubMed] [Google Scholar]
- Cooney D. A., Dalal M., Mitsuya H., McMahon J. B., Nadkarni M., Balzarini J., Broder S., Johns D. G. Initial studies on the cellular pharmacology of 2',3-dideoxycytidine, an inhibitor of HTLV-III infectivity. Biochem Pharmacol. 1986 Jul 1;35(13):2065–2068. doi: 10.1016/0006-2952(86)90571-x. [DOI] [PubMed] [Google Scholar]
- Diver J. M., Wise R. Morphological and biochemical changes in Escherichia coli after exposure to ciprofloxacin. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):31–41. doi: 10.1093/jac/18.supplement_d.31. [DOI] [PubMed] [Google Scholar]
- Elespuru R. K., Moore S. G. Micro-BIA, a colorimetric microtiter assay of lambda prophage induction. Mutat Res. 1986 Feb;164(1):31–40. doi: 10.1016/0165-1161(86)90039-7. [DOI] [PubMed] [Google Scholar]
- Elespuru R. K., White R. J. Biochemical prophage induction assay: a rapid test for antitumor agents that interact with DNA. Cancer Res. 1983 Jun;43(6):2819–2830. [PubMed] [Google Scholar]
- Elespuru R. K., Yarmolinsky M. B. A colorimetric assay of lysogenic induction designed for screening potential carcinogenic and carcinostatic agents. Environ Mutagen. 1979;1(1):65–78. doi: 10.1002/em.2860010113. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Ferone R., Freeman G. A., Fyfe J. A., Hill J. A., Ray P. H., Richards C. A., Singer S. C., Knick V. B., Rideout J. L. Antibacterial activity and mechanism of action of 3'-azido-3'-deoxythymidine (BW A509U). Antimicrob Agents Chemother. 1987 Feb;31(2):274–280. doi: 10.1128/aac.31.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Barry D. W. Spectrum of antiviral activity and mechanism of action of zidovudine. An overview. Am J Med. 1988 Aug 29;85(2A):176–181. [PubMed] [Google Scholar]
- Furman P. A., Fyfe J. A., St Clair M. H., Weinhold K., Rideout J. L., Freeman G. A., Lehrman S. N., Bolognesi D. P., Broder S., Mitsuya H. Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. P. Design of some nucleic acid antimetabolites: expectations and reality. Invest New Drugs. 1989 Apr;7(1):51–57. doi: 10.1007/BF00178191. [DOI] [PubMed] [Google Scholar]
- Janion C., Myszkowska K. Mutagenic and inhibitory properties of some new purine analogs on Salmonella typhimurium TA1530. Mutat Res. 1981 May;91(3):193–197. doi: 10.1016/0165-7992(81)90030-0. [DOI] [PubMed] [Google Scholar]
- Kada T., Tutikawa K., Sadaie Y. In vitro and host-mediated "rec-assay" procedures for screening chemical mutagens; and phloxine, a mutagenic red dye detected. Mutat Res. 1972 Oct;16(2):165–174. doi: 10.1016/0027-5107(72)90177-7. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Mamber S. W., Bryson V., Katz S. E. The Escherichia coli WP2/WP100 rec assay for detection of potential chemical carcinogens. Mutat Res. 1983 Feb;119(2):135–144. doi: 10.1016/0165-7992(83)90121-5. [DOI] [PubMed] [Google Scholar]
- Mamber S. W., Okasinski W. G., Pinter C. D., Tunac J. B. The Escherichia coli K-12 SOS chromotest agar spot test for simple, rapid detection of genotoxic agents. Mutat Res. 1986 Aug-Sep;171(2-3):83–90. doi: 10.1016/0165-1218(86)90039-x. [DOI] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Matthes E., Lehmann C., Scholz D., von Janta-Lipinski M., Gaertner K., Rosenthal H. A., Langen P. Inhibition of HIV-associated reverse transcriptase by sugar-modified derivatives of thymidine 5'-triphosphate in comparison to cellular DNA polymerases alpha and beta. Biochem Biophys Res Commun. 1987 Oct 14;148(1):78–85. doi: 10.1016/0006-291x(87)91078-3. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1911–1915. doi: 10.1073/pnas.83.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Strategies for antiviral therapy in AIDS. 1987 Feb 26-Mar 4Nature. 325(6107):773–778. doi: 10.1038/325773a0. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Nakamura N., Moriya M., Shirasu Y., Kada T. The SOS-function-inducing activity of chemical mutagens in Escherichia coli. Mutat Res. 1984 Mar-Apr;131(3-4):101–109. doi: 10.1016/0167-8817(84)90048-8. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Baba M., Balzarini J., Herdewijn P., Desmyter J., Robins M. J., Zou R. M., Madej D., De Clercq E. Investigations on the anti-HIV activity of 2',3'-dideoxyadenosine analogues with modifications in either the pentose or purine moiety. Potent and selective anti-HIV activity of 2,6-diaminopurine 2',3'-dideoxyriboside. Biochem Pharmacol. 1988 Apr 1;37(7):1317–1325. doi: 10.1016/0006-2952(88)90789-7. [DOI] [PubMed] [Google Scholar]
- Pietrzykowska I., Krych M., Shugar D. Induction of SOS functions in Escherichia coli by lesions resulting from incorporation of 5-bromouracil into DNA. Mutat Res. 1983 Oct;111(2):119–133. doi: 10.1016/0027-5107(83)90057-x. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Huisman O., D'Ari R., Hofnung M. SOS chromotest, a direct assay of induction of an SOS function in Escherichia coli K-12 to measure genotoxicity. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5971–5975. doi: 10.1073/pnas.79.19.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuck G., Pechan R., Wild D., Schiffmann D., Henschler D. SOS-dependent mutagenic activity of 5-azacytidine in salmonella. Mutat Res. 1986 Dec;175(4):205–208. doi: 10.1016/0165-7992(86)90055-2. [DOI] [PubMed] [Google Scholar]
- St Clair M. H., Richards C. A., Spector T., Weinhold K. J., Miller W. H., Langlois A. J., Furman P. A. 3'-Azido-3'-deoxythymidine triphosphate as an inhibitor and substrate of purified human immunodeficiency virus reverse transcriptase. Antimicrob Agents Chemother. 1987 Dec;31(12):1972–1977. doi: 10.1128/aac.31.12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toji L., Cohen S. S. Termination of deoxyribonucleic acid in Escherichia coli by 2',3'-dideoxyadenosine. J Bacteriol. 1970 Aug;103(2):323–328. doi: 10.1128/jb.103.2.323-328.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toji L., Cohen S. S. The enzymatic termination of polydeoxynucleotides by 2',3'-dideoxyadenosine triphosphate. Proc Natl Acad Sci U S A. 1969 Jul;63(3):871–877. doi: 10.1073/pnas.63.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweats D. J., Green M. H., Muriel W. J. A differential killing assay for mutagens and carcinogens based on an improved repair-deficient strain of Escherichia coli. Carcinogenesis. 1981;2(3):189–194. doi: 10.1093/carcin/2.3.189. [DOI] [PubMed] [Google Scholar]
- Vrang L., Bazin H., Remaud G., Chattopadhyaya J., Oberg B. Inhibition of the reverse transcriptase from HIV by 3'-azido-3'-deoxythymidine triphosphate and its threo analogue. Antiviral Res. 1987 Mar;7(3):139–149. doi: 10.1016/0166-3542(87)90002-7. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Hutchinson F. Response to bleomycin of Escherichia coli mutants deficient in DNA repair. J Antibiot (Tokyo) 1979 Nov;32(11):1181–1185. doi: 10.7164/antibiotics.32.1181. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Hutchinson F. The effect of bleomycin on DNA in Escherichia coli K12 cells. Chem Biol Interact. 1984 Sep 15;51(2):233–246. doi: 10.1016/0009-2797(84)90032-2. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Mitsuya H., Thomas R. V., Pluda J. M., Hartman N. R., Perno C. F., Marczyk K. S., Allain J. P., Johns D. G., Broder S. In vivo activity against HIV and favorable toxicity profile of 2',3'-dideoxyinosine. Science. 1989 Jul 28;245(4916):412–415. doi: 10.1126/science.2502840. [DOI] [PubMed] [Google Scholar]


