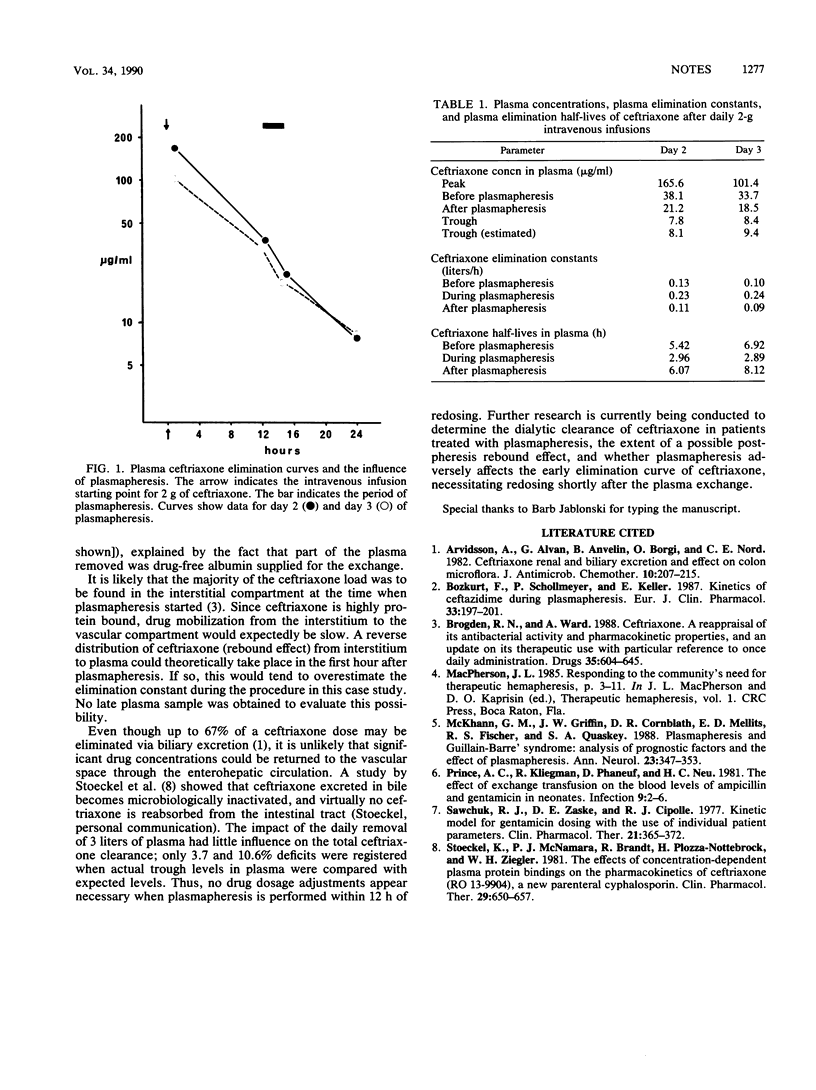
Abstract
The plasma elimination rates of serial 2-g intravenous injections of ceftriaxone were studied in a patient who also was treated with therapeutic plasma exchange pheresis. Plasmapheresis had negligible influence on the total clearance of ceftriaxone.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson A., Alván G., Angelin B., Borgå O., Nord C. E. Ceftriaxone: renal and biliary excretion and effect on the colon microflora. J Antimicrob Chemother. 1982 Sep;10(3):207–215. doi: 10.1093/jac/10.3.207. [DOI] [PubMed] [Google Scholar]
- Bozkurt F., Schollmeyer P., Keller E. Kinetics of ceftazidime during plasmapheresis. Eur J Clin Pharmacol. 1987;33(2):197–201. doi: 10.1007/BF00544567. [DOI] [PubMed] [Google Scholar]
- Brogden R. N., Ward A. Ceftriaxone. A reappraisal of its antibacterial activity and pharmacokinetic properties, and an update on its therapeutic use with particular reference to once-daily administration. Drugs. 1988 Jun;35(6):604–645. doi: 10.2165/00003495-198835060-00002. [DOI] [PubMed] [Google Scholar]
- McKhann G. M., Griffin J. W., Cornblath D. R., Mellits E. D., Fisher R. S., Quaskey S. A. Plasmapheresis and Guillain-Barré syndrome: analysis of prognostic factors and the effect of plasmapheresis. Ann Neurol. 1988 Apr;23(4):347–353. doi: 10.1002/ana.410230406. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]



