Abstract
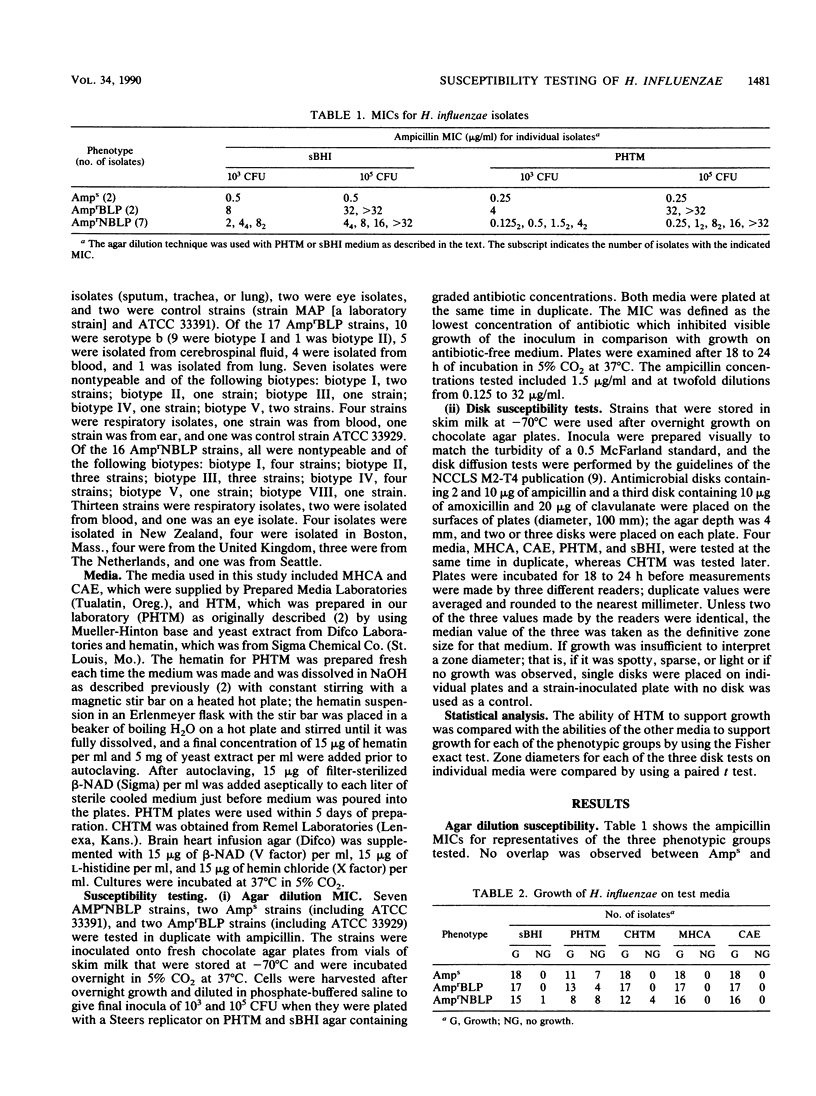
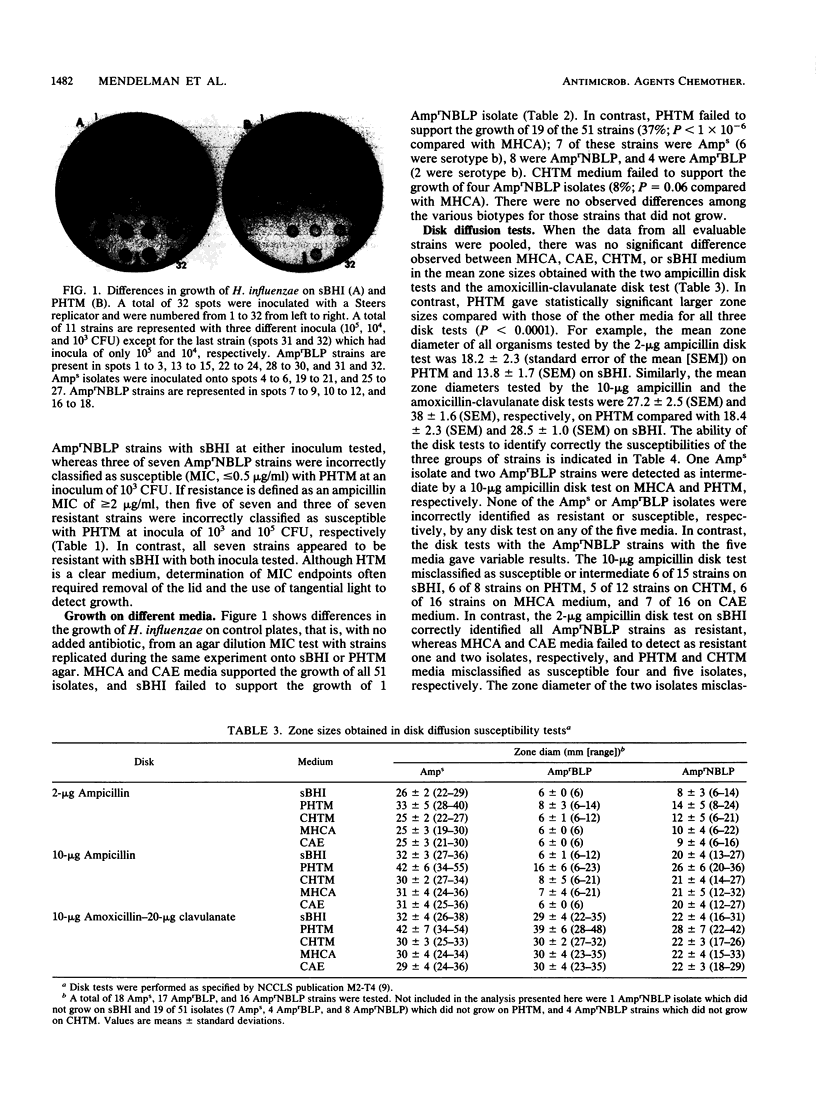
We compared results of MIC and disk susceptibility tests on Haemophilus test medium (HTM) and those on comparative media. Ampicillin MICs were determined with seven ampicillin-resistant, non-beta-lactamase-producing (AmprNBLP) isolates by using HTM and supplemented brain heart infusion (sBHI) agar. Ampicillin and amoxicillin-clavulanate disk tests with 16 AmprNBLP strains, 18 ampicillin-susceptible (Amps) isolates, and 17 ampicillin-resistant, beta-lactamase-producing (AmprBLP) strains were performed by using five media: laboratory-prepared HTM (PHTM), commercial HTM (CHTM), sBHI, enriched chocolate agar, and Mueller-Hinton chocolate agar. We observed that five of seven and three of seven AmprNBLP strains were misclassified as susceptible with PHTM (MIC, less than 2 micrograms/ml) with inocula of 10(3) and 10(5) CFU, respectively, but were resistant with sBHI (MIC, greater than or equal to 2 micrograms/ml). Whereas Mueller-Hinton chocolate agar and enriched chocolate agar plates supported the growth of all 51 strains by the disk tests, 37% (19 of 51) and 8% (4 of 51) of strains did not grow on PHTM and CHTM, respectively. Lack of growth on PHTM was observed for all three phenotypes; 7 of 18 Amps, 4 of 17 AmprBLP, and 8 of 16 AmprNBLP strains did not grow. The four strains that did not grow on CHTM were all AmprNBLP isolates. Zone sizes were significantly larger on PHTM than on the other media. Of the strains that were evaluable by the new National Committee for Clinical Laboratory Standards guidelines with either PHTM or CHTM, all Amps strains were classified as susceptible. Among the AmprBLP strains, CHTM correctly identified all as resistant, whereas PHTM detected two isolates to be intermediate. Among the AmprNBLP strains, CHTM and PHTM misclassified four (33%) and five (62%) isolates, respectively, as susceptible; an additional isolate was identified as intermediate on both media. We conclude that there is strain-dependent growth on HTM, that adoption of this medium for routine Haemophilus susceptibility testing is problematic due to this growth variability, and that detection of AmprNBLP isolates would be unreliable.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doern G. V., Jones R. N. Antimicrobial susceptibility testing of Haemophilus influenzae, Branhamella catarrhalis, and Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1988 Dec;32(12):1747–1753. doi: 10.1128/aac.32.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Redding J. S., Maher L. A., Howell A. W. Improved medium for antimicrobial susceptibility testing of Haemophilus influenzae. J Clin Microbiol. 1987 Nov;25(11):2105–2113. doi: 10.1128/jcm.25.11.2105-2113.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Clausen C., Stull T. L., Needham C., Williams J. D., Smith A. L. Failure to detect ampicillin-resistant, non-beta-lactamase-producing Haemophilus influenzae by standard disk susceptibility testing. Antimicrob Agents Chemother. 1986 Aug;30(2):274–280. doi: 10.1128/aac.30.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Musser J. M., De Groot R., Serfass D. A., Selander R. K. Genetic and phenotypic diversity among ampicillin-resistant, non-beta-lactamase-producing, nontypeable Haemophilus influenzae isolates. Infect Immun. 1987 Nov;55(11):2585–2589. doi: 10.1128/iai.55.11.2585-2589.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Henritzy L. L., Chaffin D. O., Lent K., Smith A. L., Stull T. L., Wiley E. A. In vitro activities and targets of three cephem antibiotics against Haemophilus influenzae. Antimicrob Agents Chemother. 1989 Nov;33(11):1878–1882. doi: 10.1128/aac.33.11.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]



