Abstract
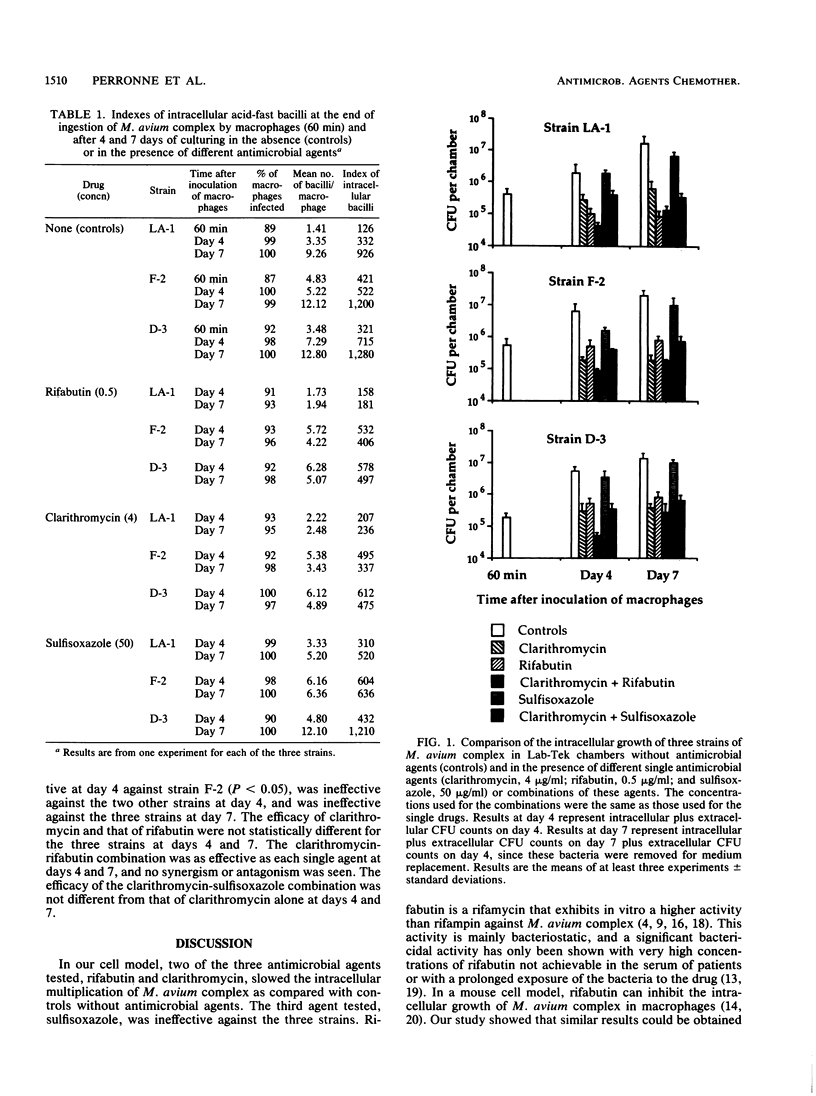
The activities of clarithromycin, sulfisoxazole, and rifabutin against three virulent strains of Mycobacterium avium complex isolated from patients with acquired immunodeficiency syndrome were evaluated in a model of intracellular infection. Human monocyte-derived macrophages were infected at day 6 of culture with M. avium complex. Intracellular bacteria were counted 60 min after inoculation. Extra- and intracellular bacteria were counted at days 4 and 7 after inoculation. The concentrations used were 4 micrograms of clarithromycin per ml (MICs for the three strains, 4, 4, and 4 micrograms/ml), 50 micrograms of sulfisoxazole per ml (MICs, 50, 25, and 25 micrograms/ml), and 0.5 micrograms of rifabutin per ml (MICs, 2, 0.5, and 0.5 micrograms/ml). Compared with controls, clarithromycin and rifabutin slowed the intracellular replication of the three strains (at day 7 after inoculation, P was less than 0.01 for the first strain and less than 0.001 for the two others). Sulfisoxazole was ineffective against the three strains. Clarithromycin was as effective as rifabutin. Clarithromycin plus rifabutin was as effective as each single agent. Clarithromycin plus sulfisoxazole was as effective as clarithromycin alone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Gold J. W., Dryjanski J., Whimbey E., Polsky B., Hawkins C., Brown A. E., Bernard E., Kiehn T. E. Treatment of infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):738–743. doi: 10.7326/0003-4819-103-5-738. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Activities of amikacin, roxithromycin, and azithromycin alone or in combination with tumor necrosis factor against Mycobacterium avium complex. Antimicrob Agents Chemother. 1988 Aug;32(8):1149–1153. doi: 10.1128/aac.32.8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Tsang A. Y., Vatter A. E., May M. H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986 Nov;24(5):812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynamon M. H. Comparative in vitro activities of MDL 473, rifampin, and ansamycin against Mycobacterium intracellulare. Antimicrob Agents Chemother. 1985 Sep;28(3):440–441. doi: 10.1128/aac.28.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D. J., McDaniel D., Hanson C. W., Swanson R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother. 1989 Sep;33(9):1531–1534. doi: 10.1128/aac.33.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Jairam B. T., Rao P. N., Nguyen A. K., Farhi D. C., Iseman M. D. Activity of rifabutin alone or in combination with clofazimine or ethambutol or both against acute and chronic experimental Mycobacterium intracellulare infections. Am Rev Respir Dis. 1987 Aug;136(2):329–333. doi: 10.1164/ajrccm/136.2.329. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Podapati N. R., Kesavalu L., Iseman M. D. In vivo activity of amikacin alone or in combination with clofazimine or rifabutin or both against acute experimental Mycobacterium avium complex infections in beige mice. Antimicrob Agents Chemother. 1988 Sep;32(9):1400–1403. doi: 10.1128/aac.32.9.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D. Determination of in vitro susceptibility of mycobacteria to ansamycin. Am Rev Respir Dis. 1985 Sep;132(3):710–711. doi: 10.1164/arrd.1985.132.3.710. [DOI] [PubMed] [Google Scholar]
- Inderlied C. B., Kolonoski P. T., Wu M., Young L. S. Amikacin, ciprofloxacin, and imipenem treatment for disseminated Mycobacterium avium complex infection of beige mice. Antimicrob Agents Chemother. 1989 Feb;33(2):176–180. doi: 10.1128/aac.33.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. J., Owens N. J., Nightingale C. H., Klimek J. J., Lehmann W. B., Quintiliani R. Penetration of amoxicillin, cefaclor, erythromycin-sulfisoxazole, and trimethoprim-sulfamethoxazole into the middle ear fluid of patients with chronic serous otitis media. J Infect Dis. 1982 Jun;145(6):815–821. doi: 10.1093/infdis/145.6.815. [DOI] [PubMed] [Google Scholar]
- Naik S., Ruck R. In vitro activities of several new macrolide antibiotics against Mycobacterium avium complex. Antimicrob Agents Chemother. 1989 Sep;33(9):1614–1616. doi: 10.1128/aac.33.9.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal V. K., Gangadharam P. R., Heifets L. B., Iseman M. D. Dynamic aspects of the in vitro chemotherapeutic activity of ansamycin (rifabutine) on mycobacterium intracellulare. Am Rev Respir Dis. 1985 Dec;132(6):1278–1280. doi: 10.1164/arrd.1985.132.6.1278. [DOI] [PubMed] [Google Scholar]
- Perumal V. K., Gangadharam P. R., Iseman M. D. Effect of rifabutin on the phagocytosis and intracellular growth of Mycobacterium intracellulare in mouse resident and activated peritoneal and alveolar macrophages. Am Rev Respir Dis. 1987 Aug;136(2):334–337. doi: 10.1164/ajrccm/136.2.334. [DOI] [PubMed] [Google Scholar]
- Skinner M. H., Hsieh M., Torseth J., Pauloin D., Bhatia G., Harkonen S., Merigan T. C., Blaschke T. F. Pharmacokinetics of rifabutin. Antimicrob Agents Chemother. 1989 Aug;33(8):1237–1241. doi: 10.1128/aac.33.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truffot-Pernot C., Giroir A. M., Maury L., Grosset J. Etude des concentrations minima inhibitrices de rifabutine (Ansamycine LM 427) pour Mycobacterium tuberculosis, Mycobacterium xenopi et Mycobacterium avium-intracellulare. Rev Mal Respir. 1988;5(4):401–406. [PubMed] [Google Scholar]
- Wollschlager C. M., Khan F. A., Chitkara R. K., Shivaram U. Pulmonary manifestations of the acquired immunodeficiency syndrome (AIDS). Chest. 1984 Feb;85(2):197–202. doi: 10.1378/chest.85.2.197. [DOI] [PubMed] [Google Scholar]
- Woodley C. L., Kilburn J. O. In vitro susceptibility of Mycobacterium avium complex and Mycobacterium tuberculosis strains to a spiro-piperidyl rifamycin. Am Rev Respir Dis. 1982 Sep;126(3):586–587. doi: 10.1164/arrd.1982.126.3.586. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Therapeutic implications of inhibition versus killing of Mycobacterium avium complex by antimicrobial agents. Antimicrob Agents Chemother. 1987 Jan;31(1):117–120. doi: 10.1128/aac.31.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Sanders C. A., Hadley W. K. Killing by antimycobacterial agents of AIDS-derived strains of Mycobacterium avium complex inside cells of the mouse macrophage cell line J774. Am Rev Respir Dis. 1989 Nov;140(5):1198–1203. doi: 10.1164/ajrccm/140.5.1198. [DOI] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]


