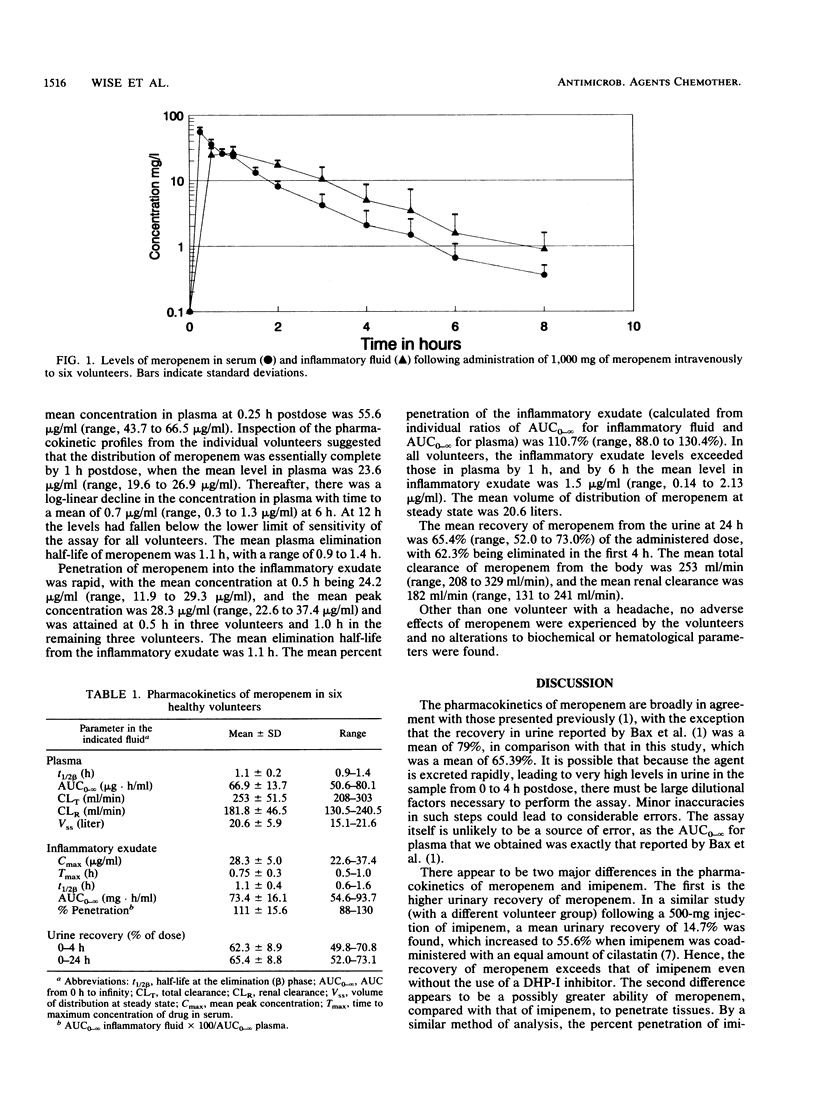
Abstract
The pharmacokinetics and penetration into a cantharidine-induced inflammatory exudate of meropenem was studied in six volunteers following a single 1-g intravenous dose. Concentrations in plasma, urine, and the inflammatory exudate were determined by a microbiological assay. The mean elimination half-life of meropenem in plasma was 1.1 h, with the concentration in plasma declining from a mean of 23.6 micrograms/ml at 1 h to 0.7 micrograms/ml at 6 h. The inflammatory fluid penetration was rapid (time to maximum concentration of drug in serum, 0.75 h), and the penetration was 111%. The recovery of meropenem in urine at 24 h was 65.4% of the administered dose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax R. P., Bastain W., Featherstone A., Wilkinson D. M., Hutchison M., Haworth S. J. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):311–320. doi: 10.1093/jac/24.suppl_a.311. [DOI] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. R., Turner P. J., Wannop C., Withnell E. S., Grindey A. J., Nairn K. In vitro antibacterial activity of SM-7338, a carbapenem antibiotic with stability to dehydropeptidase I. Antimicrob Agents Chemother. 1989 Feb;33(2):215–222. doi: 10.1128/aac.33.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeni R. PHARM--an interactive graphic program for individual and population pharmacokinetic parameter estimation. Comput Biol Med. 1984;14(1):25–34. doi: 10.1016/0010-4825(84)90017-9. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C. In-vitro studies of meropenem. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):9–29. doi: 10.1093/jac/24.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- King A., Boothman C., Phillips I. Comparative in-vitro activity of meropenem on clinical isolates from the United Kingdom. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):31–45. doi: 10.1093/jac/24.suppl_a.31. [DOI] [PubMed] [Google Scholar]
- Lockley M. R., Wise R. Pharmacology of imipenem. J Antimicrob Chemother. 1985 Oct;16(4):531–533. doi: 10.1093/jac/16.4.531. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Eliopoulos G. M., Sentochnik D. E. The carbapenems: new broad spectrum beta-lactam antibiotics. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):1–7. doi: 10.1093/jac/24.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Rogers J. D., Ferber F., Jones K. H., Zacchei A. G., Weidner L. L., Demetriades J. L., Gravallese D. A., Hsieh J. Y. Disposition of radiolabeled imipenem and cilastatin in normal human volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):707–714. doi: 10.1128/aac.26.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye K. J., Shi Y. G., Andrews J. M., Wise R. Pharmacokinetics and tissue penetration of cefepime. J Antimicrob Chemother. 1989 Jul;24(1):23–28. doi: 10.1093/jac/24.1.23. [DOI] [PubMed] [Google Scholar]
- Webberley J. M., Wise R., Andrews J. M., Ashby J. P., Wallbridge D. The pharmacokinetics and tissue penetration of FCE 22101 following intravenous and oral administration. J Antimicrob Chemother. 1988 Apr;21(4):445–450. doi: 10.1093/jac/21.4.445. [DOI] [PubMed] [Google Scholar]
- Wise R., Gillett A. P., Cadge B., Durham S. R., Baker S. The influence of protein binding upon tissue fluid levels of six beta-lactam antibiotics. J Infect Dis. 1980 Jul;142(1):77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]
- Wise R. In vitro and pharmacokinetic properties of the carbapenems. Antimicrob Agents Chemother. 1986 Sep;30(3):343–349. doi: 10.1128/aac.30.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]


