Abstract
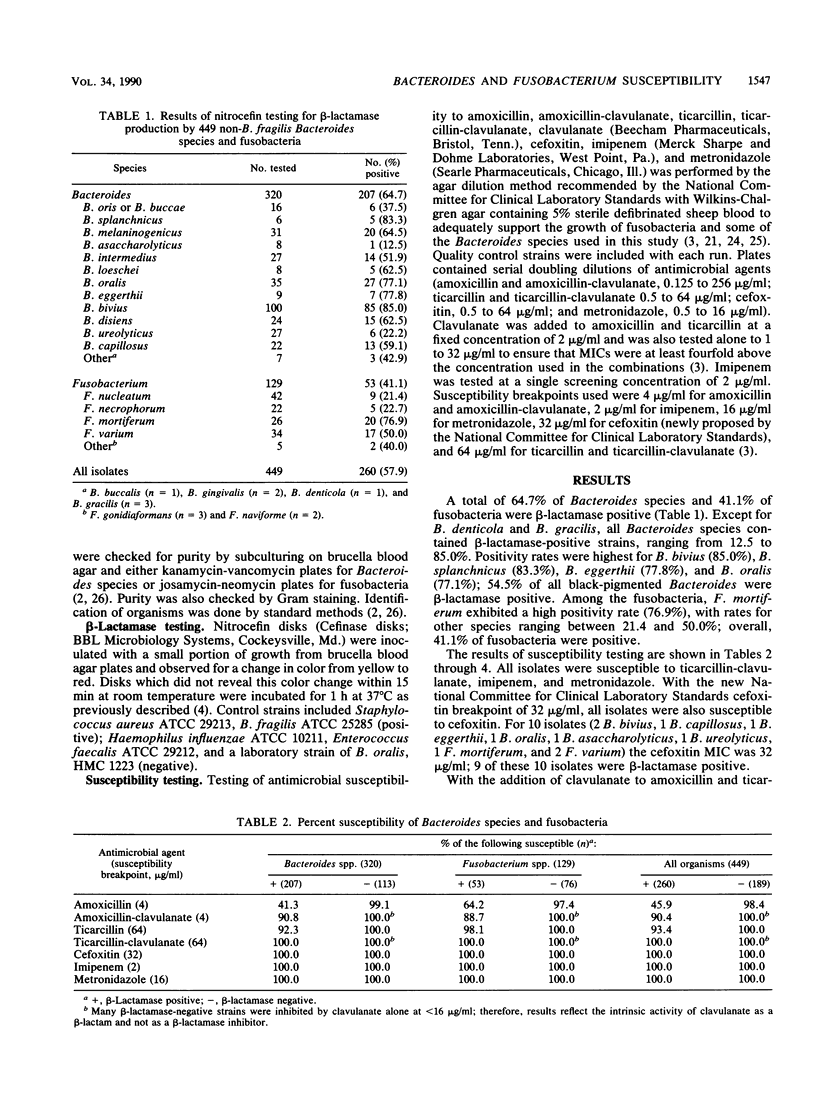
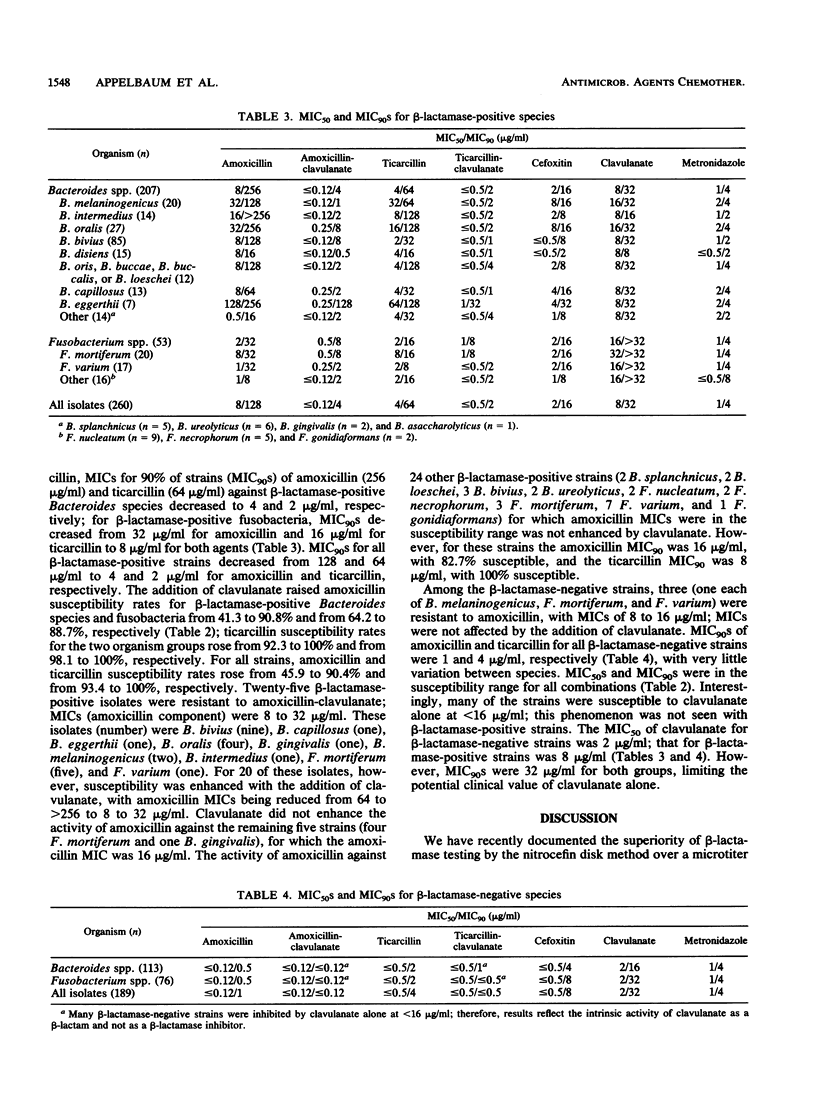
beta-Lactamase production (nitrocefin disk method) and agar dilution susceptibility of amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole were determined for 320 Bacteroides species (not Bacteroides fragilis group) and 129 fusobacteria from 28 U.S. centers. Overall, 64.7% of Bacteroides species and 41.1% of fusobacteria were beta-lactamase positive. Among the Bacteroides species, positivity rates were highest for B. bivius (85.0%), followed by B. splanchnicus (83.3%), B. eggerthii (77.8%), and B. oralis (77.1%); 54.5% of black-pigmented Bacteroides species were beta-lactamase positive. Among the fusobacteria, Fusobacterium mortiferum showed the highest rate of beta-lactamase positivity (76.9%). MICs of amoxicillin (128 micrograms/ml) and ticarcillin (64 micrograms/ml) for 90% of all beta-lactamase-positive strains were reduced to 4 and 2 micrograms/ml, respectively, with the addition of clavulanate. MICs of amoxicillin and ticarcillin for 90% of all beta-lactamase-negative strains were 1 and 4 micrograms/ml, respectively, and greater than or equal to 98.4% of the strains were susceptible to the beta-lactams tested. Of the beta-lactamase-producing strains, 45.9% were susceptible to amoxicillin at less than or equal to 4 micrograms/ml and 93.4% were susceptible to ticarcillin at less than or equal to 64 micrograms/ml; the addition of clavulanate raised the rates to 90.4 and 100%, respectively. All strains were susceptible to cefoxitin, imipenem, and metronidazole. The activity of amoxicillin against 29 beta-lactamase-producing strains (10 Bacteroides species and 19 fusobacteria) was not enhanced by the addition of clavulanate; however, 82.7% of these strains were susceptible to amoxicillin, and all were susceptible to ticarcillin. Although beta-lactamase positivity is on the increase in non-B. fragilis group Bacteroides species and fusobacteria, amoxicillin-clavulanate, ticarcillin, cefoxitin, imipenem, and metronidazole should be suitable for the treatment of infections with these strains. The addition of clavulanate does not appreciably improve the efficacy of ticarcillin against these organisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Sanders C. V., Lewis A. C., Marier R. L. Susceptibility of anaerobic bacteria to beta-lactam antibiotics and beta-lactamase production. J Med Microbiol. 1983 Feb;16(1):75–82. doi: 10.1099/00222615-16-1-75. [DOI] [PubMed] [Google Scholar]
- Appelbaum P. C., Jacobs M. R., Spangler S. K., Yamabe S. Comparative activity of beta-lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with beta-lactams against beta-lactamase-producing anaerobes. Antimicrob Agents Chemother. 1986 Nov;30(5):789–791. doi: 10.1128/aac.30.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum P. C., Spangler S. K., Jacobs M. R. Evaluation of two methods for rapid testing for beta-lactamase production in Bacteroides and Fusobacterium. Eur J Clin Microbiol Infect Dis. 1990 Jan;9(1):47–50. doi: 10.1007/BF01969535. [DOI] [PubMed] [Google Scholar]
- Bourgault A. M., Harding G. K., Smith J. A., Horsman G. B., Marrie T. J., Lamothe F. Survey of anaerobic susceptibility patterns in Canada. Antimicrob Agents Chemother. 1986 Nov;30(5):798–801. doi: 10.1128/aac.30.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I., Calhoun L., Yocum P. Beta-lactamase-producing isolates of Bacteroides species from children. Antimicrob Agents Chemother. 1980 Jul;18(1):164–166. doi: 10.1128/aac.18.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. J. National Committee for Clinical Laboratory Standards agar dilution susceptibility testing of anaerobic gram-negative bacteria. Antimicrob Agents Chemother. 1988 Mar;32(3):385–390. doi: 10.1128/aac.32.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch D. F., Kureshi L. A., Sutter V. L., Finegold S. M. Susceptibility of respiratory tract anaerobes to orally administered penicillins and cephalosporins. Antimicrob Agents Chemother. 1976 Oct;10(4):713–720. doi: 10.1128/aac.10.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby M. A., Gump D. W. Activity of cefoperazone and two beta-lactamase inhibitors, sulbactam and clavulanic acid, against Bacteroides spp. correlated with beta-lactamase production. Antimicrob Agents Chemother. 1982 Sep;22(3):398–405. doi: 10.1128/aac.22.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson R. S., Rosenblatt J. E., Lee D. T., McVey E. A., 3rd Recent experience with antimicrobial susceptibility of anaerobic bacteria: increasing resistance to penicillin. Mayo Clin Proc. 1982 Dec;57(12):737–741. [PubMed] [Google Scholar]
- George W. L., Kirby B. D., Sutter V. L., Citron D. M., Finegold S. M. Gram-negative anaerobic bacilli: Their role in infection and patterns of susceptibility to antimicrobial agents. II. Little-known Fusobacterium species and miscellaneous genera. Rev Infect Dis. 1981 May-Jun;3(3):599–626. doi: 10.1093/clinids/3.3.599. [DOI] [PubMed] [Google Scholar]
- Goldstein E. J., Citron D. M. Comparative in vitro activities of amoxicillin-clavulanic acid and imipenem against anaerobic bacteria isolated from community hospitals. Antimicrob Agents Chemother. 1986 Jan;29(1):158–160. doi: 10.1128/aac.29.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman A. S., Wilkins T. D. Influence of pencillinase production by strains of Bacteroides melaninogenicus and Bacteriodes oralis on pencillin therapy of an experimental mixed anaerobic infection in mice. Arch Oral Biol. 1976;21(6):385–389. doi: 10.1016/s0003-9969(76)80007-6. [DOI] [PubMed] [Google Scholar]
- Heimdahl A., von Konow L., Nord C. E. Beta-lactamase-producing Bacteroides species in the oral cavity in relation to penicillin therapy. J Antimicrob Chemother. 1981 Sep;8(3):225–229. doi: 10.1093/jac/8.3.225. [DOI] [PubMed] [Google Scholar]
- Heimdahl A., von Konow L., Nord C. E. Isolation of beta-lactamase-producing Bacteroides strains associated with clinical failures with penicillin treatment of human orofacial infections. Arch Oral Biol. 1980;25(10):689–692. doi: 10.1016/0003-9969(80)90102-8. [DOI] [PubMed] [Google Scholar]
- Hill G. B., Ayers O. M. Antimicrobial susceptibilities of anaerobic bacteria isolated from female genital tract infections. Antimicrob Agents Chemother. 1985 Mar;27(3):324–331. doi: 10.1128/aac.27.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby B. D., George W. L., Sutter V. L., Citron D. M., Finegold S. M. Gram-negative anaerobic bacilli: their role in infection and patterns of susceptibility to antimicrobial agents. I. Little-known Bacteroides species. Rev Infect Dis. 1980 Nov-Dec;2(6):914–951. doi: 10.1093/clinids/2.6.914. [DOI] [PubMed] [Google Scholar]
- Lacroix J. M., Lamothe F., Malouin F. Role of Bacteroides bivius beta-lactamase in beta-lactam susceptibility. Antimicrob Agents Chemother. 1984 Nov;26(5):694–698. doi: 10.1128/aac.26.5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F., Fijalkowski C., Lamothe F., Lacroix J. M. Inactivation of cefoxitin and moxalactam by Bacteroides bivius beta-lactamase. Antimicrob Agents Chemother. 1986 Nov;30(5):749–755. doi: 10.1128/aac.30.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Lindmark A., Person I. Susceptibility of anaerobic bacteria to FCE 22101. Antimicrob Agents Chemother. 1987 May;31(5):831–833. doi: 10.1128/aac.31.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm-Smith M. J., Sweet R. L. In vitro activity of cefmetazole, cefotetan, amoxicillin-clavulanic acid, and other antimicrobial agents against anaerobic bacteria from endometrial cultures of women with pelvic infections. Antimicrob Agents Chemother. 1987 Sep;31(9):1434–1437. doi: 10.1128/aac.31.9.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E. Antimicrobial susceptibility testing of anaerobic bacteria. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S242–S248. doi: 10.1093/clinids/6.supplement_1.s242. [DOI] [PubMed] [Google Scholar]
- Rowland M. D., Del Bene V. E., Lewis J. W. Factors affecting antimicrobial susceptibility of Fusobacterium species. J Clin Microbiol. 1987 Mar;25(3):476–479. doi: 10.1128/jcm.25.3.476-479.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Susceptibility of anaerobic bacteria to 23 antimicrobial agents. Antimicrob Agents Chemother. 1976 Oct;10(4):736–752. doi: 10.1128/aac.10.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. M. Rationale for identification and susceptibility testing of anaerobic bacteria. Rev Infect Dis. 1986 Sep-Oct;8(5):809–813. doi: 10.1093/clinids/8.5.809. [DOI] [PubMed] [Google Scholar]
- Timewell R. M., Phillips I., Söderholm J., Nord C. E. Isoelectric focusing of Bacteroides melaninogenicus group beta-lactamases. Antimicrob Agents Chemother. 1981 May;19(5):700–704. doi: 10.1128/aac.19.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timewell R., Taylor E., Phillips I. The beta-lactamases of Bacteroides species. J Antimicrob Chemother. 1981 Feb;7(2):137–146. doi: 10.1093/jac/7.2.137. [DOI] [PubMed] [Google Scholar]
- Tunér K., Lindqvist L., Nord C. E. Characterization of a new beta-lactamase from Fusobacterium nucleatum by substrate profiles and chromatofocusing patterns. J Antimicrob Chemother. 1985 Jul;16(1):23–30. doi: 10.1093/jac/16.1.23. [DOI] [PubMed] [Google Scholar]
- Wexler H. M., Finegold S. M. Comparative in vitro activity of the new macrolide A-56268 against anaerobic bacteria. Eur J Clin Microbiol. 1987 Aug;6(4):492–494. doi: 10.1007/BF02013121. [DOI] [PubMed] [Google Scholar]


