Abstract
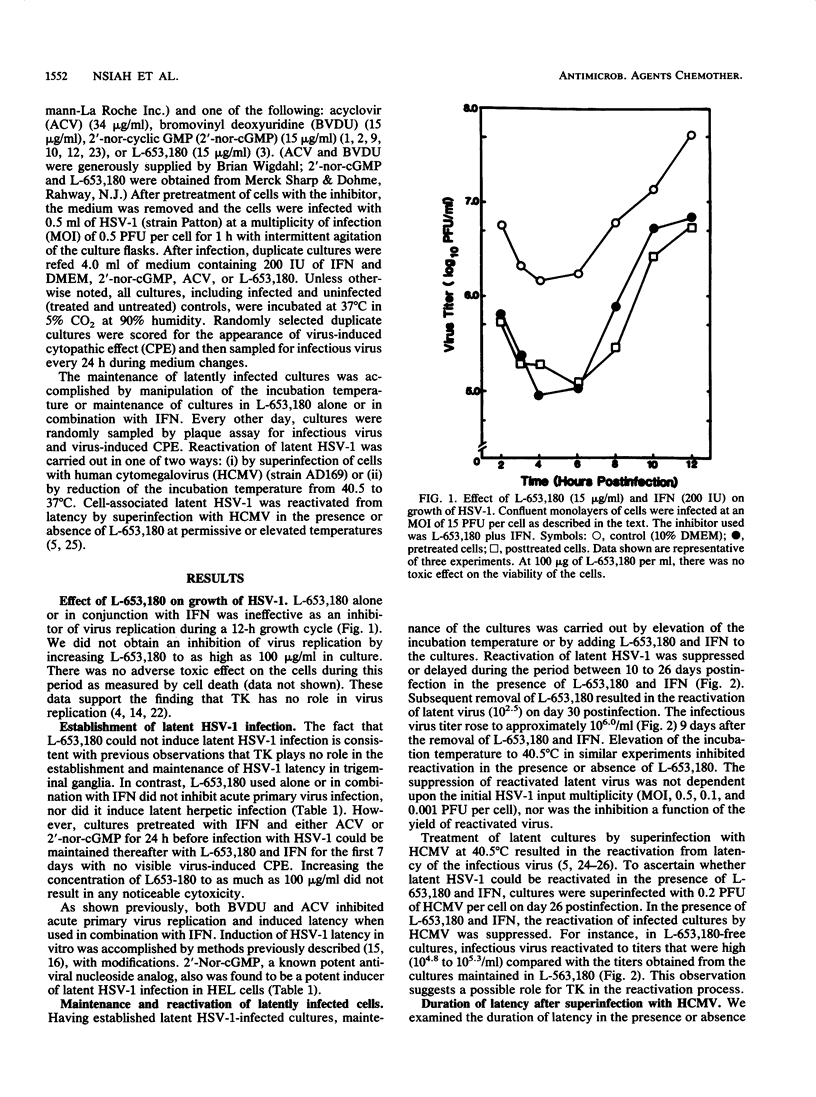
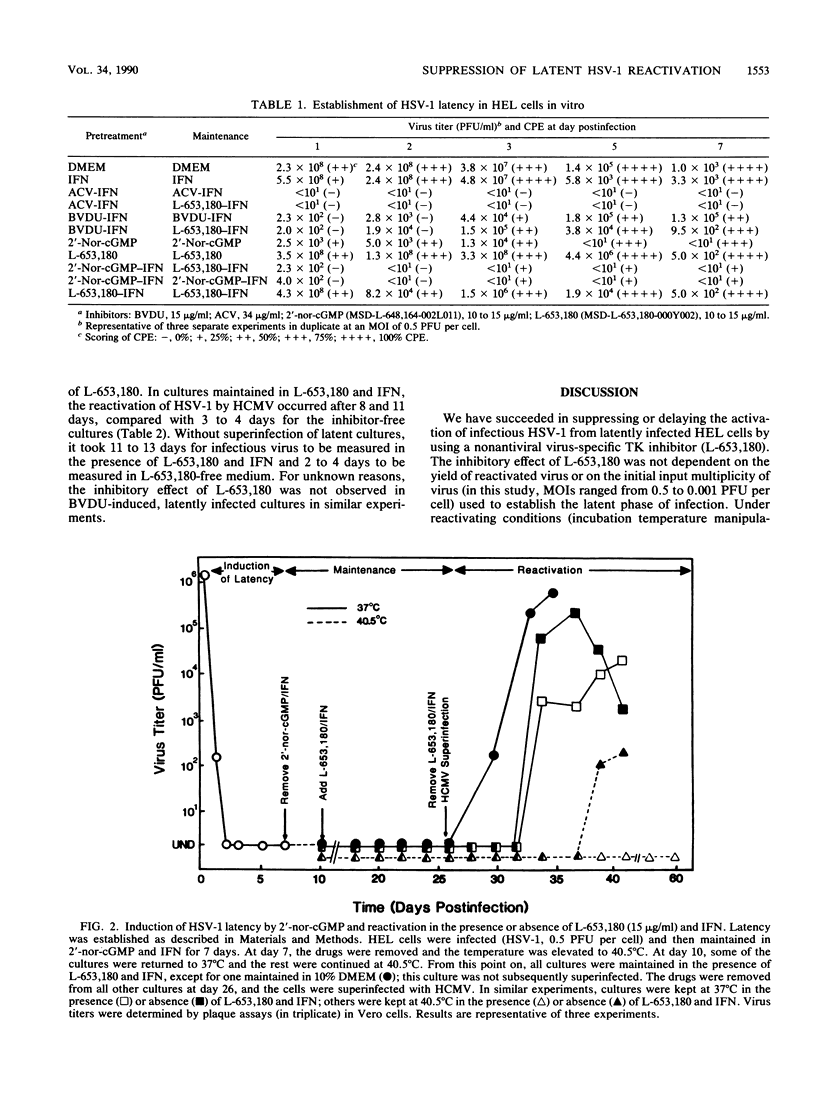
Latent herpes simplex virus type 1 (HSV-1) infection was induced in human embryonic lung cells in vitro by using a combination of viral replication inhibitors and elevated temperature. Under reactivating conditions (superinfection by human cytomegalovirus or temperature manipulation), a nonantiviral thymidine kinase inhibitor (L-653,180) was found to suppress or delay reactivation of HSV-1 from latently infected human embryonic lung cells. L-653,180 alone or in combination with interferon was ineffective as a primary or acute viral replication inhibitor and was unable to induce latent HSV-1 infection in cell culture. These data suggest that initial or acute virus replication and replication resulting from reactivation from latency are separate events.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton W. T., Canning L. F., Reynolds G. F., Tolman R. L., Karkas J. D., Liou R., Davies M. E., DeWitt C. M., Perry H. C., Field A. K. Synthesis and antiherpetic activity of (S)-, (R)-, and (+/-)-9-[(2,3-dihydroxy-1-propoxy)methyl]guanine, linear isomers of 2'-nor-2'-deoxyguanosine. J Med Chem. 1985 Jul;28(7):926–933. doi: 10.1021/jm00145a014. [DOI] [PubMed] [Google Scholar]
- Ashton W. T., Karkas J. D., Field A. K., Tolman R. L. Activation by thymidine kinase and potent antiherpetic activity of 2'-nor-2'-deoxyguanosine (2'NDG). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1716–1721. doi: 10.1016/s0006-291x(82)80109-5. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Kosz-Vnenchak M., Jacobson J. G., Leib D. A., Bogard C. L., Schaffer P. A., Tyler K. L., Knipe D. M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Isom H. C., Rapp F. Reactivation of herpes simplex virus type 2 from a quiescent state by human cytomegalovirus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5948–5951. doi: 10.1073/pnas.76.11.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Latent herpetic infections following experimental viraemia. J Gen Virol. 1976 Apr;31(1):75–80. doi: 10.1099/0022-1317-31-1-75. [DOI] [PubMed] [Google Scholar]
- Elion G. B. Mechanism of action and selectivity of acyclovir. Am J Med. 1982 Jul 20;73(1A):7–13. doi: 10.1016/0002-9343(82)90055-9. [DOI] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C. M., Perry H. C., Schofield T. L., Karkas J. D., Germershausen J., Wagner A. F., Cantone C. L., MacCoss M. Efficacy of 2'-nor-cyclicGMP in treatment of experimental herpes virus infections. Antiviral Res. 1986 Oct;6(6):329–341. doi: 10.1016/0166-3542(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Marsden H. S., Cook M. L., Stevens J. G. Acute infection of differentiated neuroblastoma cells by latency-positive and latency-negative herpes simplex virus ts mutants. Virology. 1979 Apr 30;94(2):430–441. doi: 10.1016/0042-6822(79)90473-2. [DOI] [PubMed] [Google Scholar]
- Germershausen J., Bostedor R., Liou R., Field A. K., Wagner A. F., MacCoss M., Tolman R. L., Karkas J. D. Comparison of the modes of antiviral action of 2'-nor-deoxyguanosine and its cyclic phosphate, 2'-nor-cyclic GMP. Antimicrob Agents Chemother. 1986 Jun;29(6):1025–1031. doi: 10.1128/aac.29.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J. Initiation and maintenance of latent herpes simplex virus infections: the paradox of perpetual immobility and continuous movement. Rev Infect Dis. 1985 Jan-Feb;7(1):21–30. doi: 10.1093/clinids/7.1.21. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Sandri-Goldin R. M., Stevens J. G. Latent infections in spinal ganglia with thymidine kinase-deficient herpes simplex virus. J Virol. 1989 Nov;63(11):4976–4978. doi: 10.1128/jvi.63.11.4976-4978.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Goldin A. L., Glorioso J. C. Persistence of herpes simplex virus genes in cells of neuronal origin. J Virol. 1980 Jul;35(1):203–210. doi: 10.1128/jvi.35.1.203-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter L. M., Grill S. P., Dutschman G. E., Sharma R. A., Bobek M., Cheng Y. C. Demonstration of viral thymidine kinase inhibitor and its effect on deoxynucleotide metabolism in cells infected with herpes simplex virus. Antimicrob Agents Chemother. 1987 Mar;31(3):368–374. doi: 10.1128/aac.31.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill F. J., Goldberg R. J., Rapp F. Herpes simplex virus latency in cultured human cells following treatment with cytosine arabinoside. J Gen Virol. 1972 Feb;14(2):189–197. doi: 10.1099/0022-1317-14-2-189. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J. Prolongation of herpes simplex virus latency in cultured human cells by temperature elevation. J Virol. 1977 Oct;24(1):41–46. doi: 10.1128/jvi.24.1.41-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Scheck A. C., Wigdahl B., De Clercq E., Rapp F. Prolonged herpes simplex virus latency in vitro after treatment of infected cells with acyclovir and human leukocyte interferon. Antimicrob Agents Chemother. 1986 Apr;29(4):589–593. doi: 10.1128/aac.29.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Hay K. A., Edris W. A. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J Virol. 1989 Jun;63(6):2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman R. L., Field A. K., Karkas J. D., Wagner A. F., Germershausen J., Crumpacker C., Scolnick E. M. 2'-Nor-cGMP: a seco-cyclic nucleotide with powerful anti-DNA-viral activity. Biochem Biophys Res Commun. 1985 May 16;128(3):1329–1335. doi: 10.1016/0006-291x(85)91086-1. [DOI] [PubMed] [Google Scholar]
- Wigdahl B. L., Isom H. C., Rapp F. Repression and activation of the genome of herpes simplex viruses in human cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6522–6526. doi: 10.1073/pnas.78.10.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigdahl B. L., Scheck A. C., De Clercq E., Rapp F. High efficiency latency and activation of herpes simplex virus in human cells. Science. 1982 Sep 17;217(4565):1145–1146. doi: 10.1126/science.6180477. [DOI] [PubMed] [Google Scholar]
- Wigdahl B., Scheck A. C., Ziegler R. J., De Clercq E., Rapp F. Analysis of the herpes simplex virus genome during in vitro latency in human diploid fibroblasts and rat sensory neurons. J Virol. 1984 Jan;49(1):205–213. doi: 10.1128/jvi.49.1.205-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzos H., Rapp F. Establishment of latency in vitro with herpes simplex virus temperature-sensitive mutants at nonpermissive temperature. Virus Res. 1987 Nov;8(4):301–308. doi: 10.1016/0168-1702(87)90002-5. [DOI] [PubMed] [Google Scholar]


