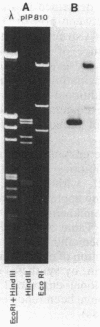
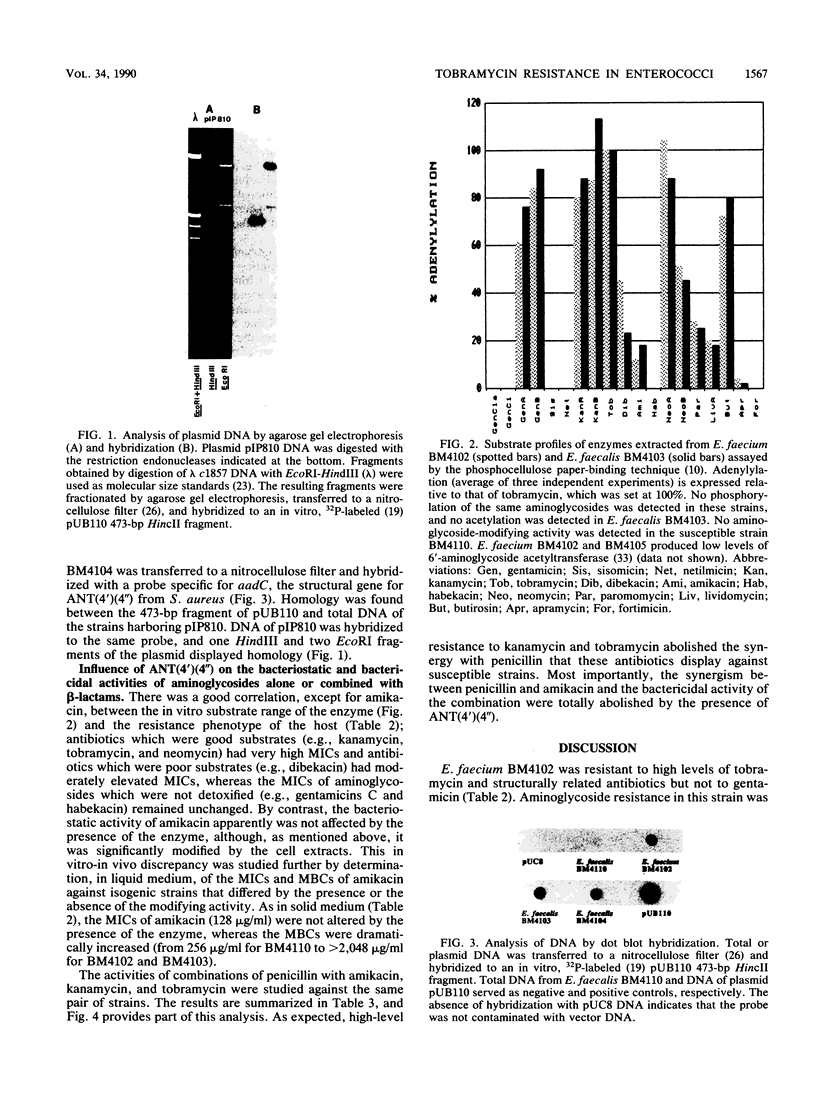
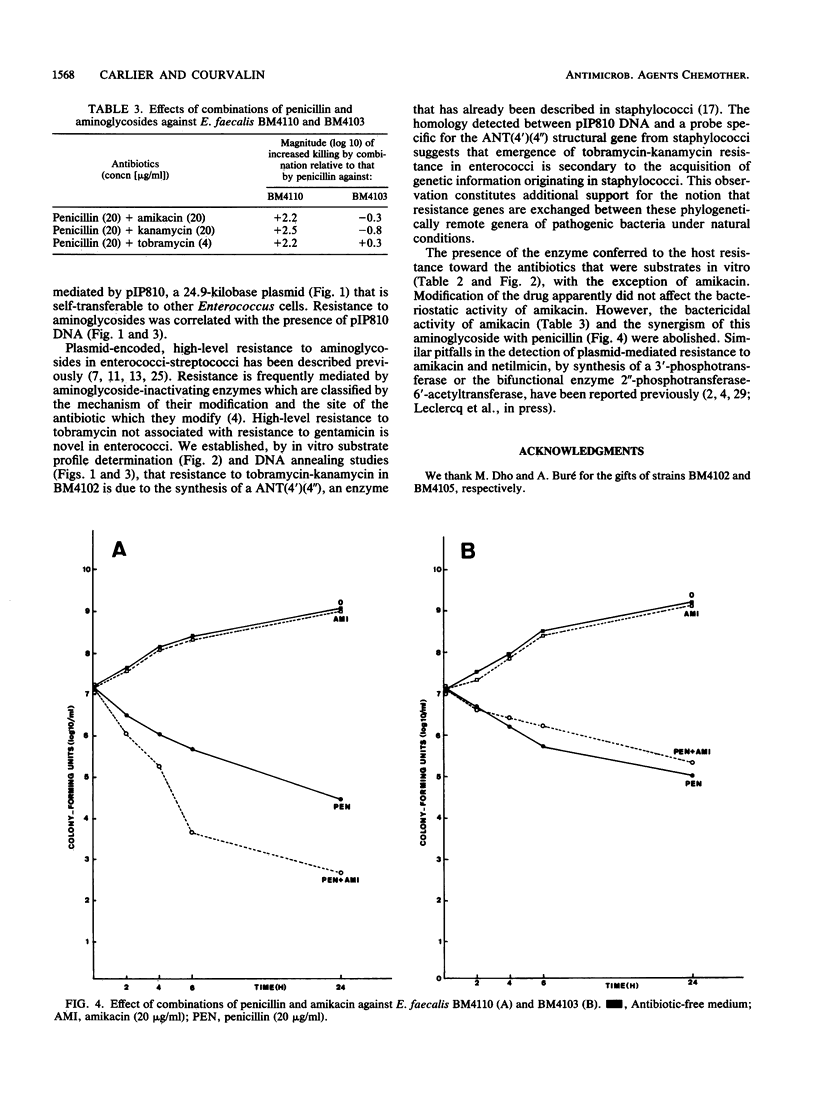
Abstract
Enterococcus faecium BM4102 was resistant to macrolide-lincosamide-streptogramin B-type (MLS) antibiotics; tetracycline-minocycline; and high levels of kanamycin, neomycin, tobramycin, and dibekacin but not gentamicin. This aminoglycoside resistance phenotype is new in enterococci. The genes conferring resistance to aminoglycosides and MLS antibiotics in this strain were carried on a plasmid, pIP810, that was self-transferable to to other Enterococcus strains. Resistance to tobramycin and structurally related aminoglycosides, kanamycin, neomycin, and dibekacin, was due to synthesis of a 4',4"-aminoglycoside nucleotidyltransferase. Homology was detected by hybridization between pIP810 DNA and a probe specific for a gene encoding an enzyme with identical site specificity in staphylococci. The bacteriostatic activity of amikacin apparently was not affected by the presence of the enzyme, although it was modified in vitro. However, the bactericidal activity of amikacin and the synergism of this aminoglycoside with penicillin were abolished.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Wennersten C., Moellering R. C., Jr Resistance to antibiotic synergism in Streptococcus faecalis: further studies with amikacin and with a new amikacin derivative, 4'-deoxy, 6'-N-methylamikacin. Antimicrob Agents Chemother. 1981 Apr;19(4):549–555. doi: 10.1128/aac.19.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Shaw W. V., Jacob A. E. Plasmid-mediated mechanisms of resistance to aminoglycoside-aminocyclitol antibiotics and to chloramphenicol in group D streptococci. Antimicrob Agents Chemother. 1978 May;13(5):716–725. doi: 10.1128/aac.13.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Resistance towards aminoglycoside-aminocyclitol antibiotics in bacteria. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):57–69. doi: 10.1093/jac/8.suppl_a.57. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol Gen Genet. 1986 Nov;205(2):291–297. doi: 10.1007/BF00430441. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Aminoglycoside-inactivating enzymes in clinical isolates of Streptococcus faecalis. An explanation for resistance to antibiotic synergism. J Clin Invest. 1978 Aug;62(2):480–486. doi: 10.1172/JCI109149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Plasmid-mediated resistance to antibiotic synergism in enterococci. J Clin Invest. 1978 Jun;61(6):1645–1653. doi: 10.1172/JCI109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Pargwette A. R. Defective killing of enterococci: a common property of antimicrobial agents acting on the cell wall. Antimicrob Agents Chemother. 1980 Jun;17(6):965–968. doi: 10.1128/aac.17.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Chopra I. Genetic studies of a multi-resistant strain of Staphylococcus aureus. J Med Microbiol. 1974 May;7(2):285–297. doi: 10.1099/00222615-7-2-285. [DOI] [PubMed] [Google Scholar]
- Le Goffic F., Baca B., Soussy C. J., Dublanchet A., Duval J. ANT(4')I: une nouvelle nucléotidyltransférase d'aminoglycosides isolée de Staphylococcus aureus. Ann Microbiol (Paris) 1976 Apr;127(3):391–399. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M., Katakura Y., Imanaka T., Aiba S. Enzymatic and nucleotide sequence studies of a kanamycin-inactivating enzyme encoded by a plasmid from thermophilic bacilli in comparison with that encoded by plasmid pUB110. J Bacteriol. 1984 Oct;160(1):413–420. doi: 10.1128/jb.160.1.413-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Korzeniowski O. M., Sande M. A., Wennersten C. B. Species-specific resistance to antimocrobial synergism in Streptococcus faecium and Streptococcus faecalis. J Infect Dis. 1979 Aug;140(2):203–208. doi: 10.1093/infdis/140.2.203. [DOI] [PubMed] [Google Scholar]
- Sadaie Y., Burtis K. C., Doi R. H. Purification and characterization of a kanamycin nucleotidyltransferase from plasmid pUB110-carrying cells of Bacillus subtilis. J Bacteriol. 1980 Mar;141(3):1178–1182. doi: 10.1128/jb.141.3.1178-1182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Standiford H. D., De Maine J. B., Kirby W. M. Antibiotic synergism of enterococci. Relation to inhibitory concentrations. Arch Intern Med. 1970 Aug;126(2):255–259. [PubMed] [Google Scholar]
- Thauvin C., Eliopoulos G. M., Wennersten C., Moellering R. C., Jr Antagonistic effect of penicillin-amikacin combinations against enterococci. Antimicrob Agents Chemother. 1985 Jul;28(1):78–83. doi: 10.1128/aac.28.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Carlier C., Courvalin P. Conjugative plasmid transfer from Enterococcus faecalis to Escherichia coli. J Bacteriol. 1988 Sep;170(9):4388–4391. doi: 10.1128/jb.170.9.4388-4391.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. Penicillin combined with gentamicin or streptomycin: synergism against enterococci. J Infect Dis. 1971 Dec;124(6):581–586. doi: 10.1093/infdis/124.6.581. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Moellering R. C., Jr, Weinberg A. N. Mechanism of resistance to antibiotic synergism in enterococci. J Bacteriol. 1971 Mar;105(3):873–879. doi: 10.1128/jb.105.3.873-879.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]