Abstract
Nucleotide excision repair (NER), apoptosis, and cell-cycle regulation are major defense mechanisms against the carcinogenic effects of UVB light. NER eliminates UVB-induced DNA photolesions via two subpathways: global genome repair (GGR) and transcription-coupled repair (TCR). Defects in NER result in the human disorders xeroderma pigmentosum (XP) and Cockayne syndrome (CS), displaying severe UV sensitivity and in the case of XP, cancer proneness. We investigated the impact of deficiencies in NER subpathways on apoptosis, hyperplasia, and cell cycle progression in the epidermis of UVB-exposed CS group B (Csb−/−) mice (no TCR), XP group C (Xpc−/−) mice (no GGR), and XP group A (Xpa−/−) mice (no TCR and no GGR). On UVB treatment (250 J/m2), Xpa−/− and Csb−/− mice revealed an extensive apoptotic response in the skin, a blockage of cell cycle progression of epidermal cells, and strong hyperplasia. Interestingly, the absence of this apoptotic response in the skin of wild-type and Xpc−/− mice coincided with the ability of epidermal cells to enter the S phase. However, only epidermal cells of Xpc−/− mice subsequently became arrested in the G2 phase. Our data demonstrate that TCR (and/or restoration of UVB-inhibited transcription) enables damaged cells to progress through S phase and prevents the induction of apoptosis and hyperplasia. G2 arrest is manifest only under conditions of proficient TCR in combination with deficient GGR, indicating that epidermal cells become arrested in the G2 phase as a result of persisting damage in their genome.
Nucleotide excision repair (NER) is a versatile DNA repair mechanism that eliminates a wide variety of DNA lesions, including DNA photolesions caused by exposure of cells to UVB light—e.g., cyclobutane pyrimidine dimers (CPD) and pyrimidine(6–4)pyrimidone photoproducts (6–4PP). Two NER subpathways have been identified: global genome repair (GGR) eliminates lesions in the bulk of the DNA, whereas transcription-coupled repair (TCR) removes lesions from RNA polymerase II-transcribed DNA strands (1, 2).
Xeroderma pigmentosum (XP) and Cockayne syndrome (CS) are rare genetic human disorders characterized by a high sensitivity to sunlight, resulting in sunburn at relatively low levels of exposure (3, 4). The hallmarks for sunburn are cutaneous vasodilatation (erythema) and exudation of fluid (edema) in the irradiated skin. In addition, XP patients have an increased predisposition to skin cancer in sun-exposed areas (3, 5, 6). Cell fusion studies have identified seven XP complementation groups (A through G) and two CS complementation groups (A and B) (4, 7). In general, cells from XP patients show an overall NER defect—i.e., both GGR and TCR are defective, with the exception of XP-C cells, which are GGR defective only (8, 9). Cells from CS patients are GGR proficient, but TCR defective (10, 11).
The mechanisms that lead to acute effects in the skin after UVB irradiation—e.g., erythema and edema—are unclear. Experiments to elucidate these mechanisms are difficult to perform in humans for obvious ethical reasons. NER-deficient mouse models have been proven valuable tools to study acute and long-term biological effects of UVB light. Phenotypically, these mouse models show the same repair phenotype as their human counterparts: XP group A (Xpa−/−) mice have an overall NER defect (12, 13), XP group C (Xpc−/−) mice are defective in GGR (14, 15), and CS group B (Csb−/−) mice in TCR (16). Like humans, both Xpa−/− and Xpc−/− mice show an increased susceptibility to UVB-induced nonmelanoma skin cancer (12, 13, 17–19). Although not reported for CS patients, Csb−/− mice develop UV-induced skin carcinomas, albeit at a lower rate than Xpa−/− and Xpc−/− mice (16). Xpa−/− and Csb−/− mice have an increased susceptibility to sunburn (12, 13, 16, 19, 20) in contrast to Xpc−/− mice (15), suggesting that TCR is important to prevent sunburn.
Besides NER, several other cellular processes counteract the detrimental effects of DNA damage. Cells with damaged DNA can be arrested in certain stages of the cell cycle and undergo apoptosis, thereby preventing the replication of damaged DNA (G1/S arrest) or mitotic division of a damaged genome (G2 arrest). Transient cell cycle arrest allows repair of DNA damage, after which cell cycle progression can be resumed. DNA repair and apoptosis are mechanisms that reduce the load of DNA damage and the number of damaged cells, respectively.
In the present study, we investigated the impact of NER subpathways on cell cycle progression, apoptosis, and hyperplasia in the epidermis of UVB-exposed mice. The results show a correlation between impaired TCR and enhanced sensitivity to UVB-induced apoptosis, indicating that TCR protects against UVB-induced apoptosis (sunburn cells). Absence of an apoptotic response in the epidermis of TCR-proficient mice correlates with the ability of epidermal cells to enter S phase. Furthermore, our experiments reveal a UVB-induced G2 arrest unique for mice expressing TCR in the absence of GGR (i.e., Xpc−/− mice).
Materials and Methods
Transgenic Mice.
The generation of Xpa−/−, Xpc−/−, and Csb−/− mice has been described (12, 14, 16). All three mouse strains were crossed with albino hairless mice (SKH-1) and the offspring were backcrossed with SKH-1 (Charles River Breeding Laboratories). The resulting mice were intercrossed to obtain hairless wild-type (WT), homozygous NER-deficient, and heterozygous littermates. The heterozygous and WT littermates were used as controls for the NER-deficient mice.
Animals were kept individually with standard mouse chow and water available ad libitum in a room illuminated with yellow light (no measurable UV) in a 12-h cycle.
UV Dosimetry and Irradiation.
To compare cell cycle progression and apoptosis of epidermal cells with previously reported data on acute skin effects (edema and erythema) and skin carcinogenesis in UVB-irradiated mice (19, 20), it is essential to use the same UVB radiation regimen in all experiments. The previously described studies on effects of chronic exposure to UVB were performed with American Philips F40 sunlamps. Because these lamps are no longer available, cell cycle and apoptosis experiments were performed with a new series of UVB lamps (Philips TL12/40W lamps). Comparison of the two lamps revealed that the Philips TL12/40W lamps induced the same biological effects in mice at half the dose needed with the F40 lamps. The difference is caused by a shift to shorter wavelengths in the emission spectrum of the TL12/40W lamps compared with that of the F40 lamps. The irradiation setup used in the experiments was identical to that described (19). In the present experiments, Xpc−/− and WT mice received UVB doses of 40, 250, 500, and 2,000 J/m2. Xpa−/− and Csb−/− mice have a minimal erythema dose that is 10 times lower than the minimal erythema dose of Xpc−/− and WT mice (19, 20). For ethical reasons, Xpa−/− and Csb−/− mice received UVB doses of 40 and 250 J/m2 only. Permission for the experiments was granted by the ethical committee of Utrecht University.
Isolation of Skin Samples and Keratinocytes.
Mice were irradiated with a single UVB dose of 40, 250, 500, or 2,000 J/m2 and killed at various times after irradiation. One hour before sacrifice, mice received an i.p. injection of 5-bromodeoxyuridine (BrdUrd; 5 mg in 300 μl of PBS, pH 7.4). Part of the middorsal skin (0.5 × 0.5 cm each) was snap frozen in liquid nitrogen. In addition, two strips of middorsal skin were excised. Epidermis and dermis were separated by overnight trypsinization at 4°C. Cell suspensions were obtained by ultrasonic vibration of the epidermal sheet in 5% newborn calf serum in PBS on ice. Cells were fixed in 70% ethanol and stored at −20°C until further use.
Dual BrdUrd/DNA Flow Cytometric Cell Cycle Analysis.
The simultaneous analysis of BrdUrd incorporation and DNA content by using flow cytometry was performed as described (21). In short, 105 ethanol-fixed cells were hydrolyzed in 2 M HCl/0.02% pepsin. Cells were incubated with monoclonal mouse-anti-BrdUrd antibody (Dakopatts, Copenhagen, Denmark) for 1 h at room temperature (RT) and subsequently incubated with FITC-conjugated rabbit anti-mouse IgG antibody (Dakopatts) for 1 h. Cells were resuspended in PBS containing propidium iodide; 104 cells were analyzed for DNA content and BrdUrd incorporation on a FACScan flow cytometer (Becton Dickinson, Immunocytometry Systems, Mountain View, CA). Data analysis was performed as described (22) by using pc-lysys software (Becton Dickinson).
Determination of Apoptotic Keratinocytes in Skin Biopsies.
Slices (5 μm) were cut from snap-frozen dorsal skin biopsies and placed on slides. Air-dried slides were fixed in acetone (H2O free) at RT for 10 min. Slides were blocked with PBS containing 5% normal mouse serum and 5% normal goat serum for 20 min at RT and incubated (RT) with polyclonal rabbit anti-active caspase 3 antibody (PharMingen) diluted 1:100 in blocking solution. After 1 h, the slides were washed with PBS/0.05% Tween 20 followed by an incubation for 1 h with biotin-conjugated goat anti-rabbit IgG antibody (Vector, Burlingame, CA) diluted 1:300 in blocking solution (RT). The slides were washed and incubated with alkaline phosphatase-conjugated ABC kit (Vectastain Elite, Vector Laboratories) (1:100) for 45 min at RT. Slides were stained with New Fuchsin and counterstained with Mayer's hematoxylin.
The extent of apoptosis was quantified as the number of apoptotic cells per arbitrary unit. Arbitrary units were chosen as a certain length along the epidermis of 5-μm sections from skin biopsy of 0.5 cm × 0.5 cm cut with the cutting edge perpendicular to the surface of the epidermis. Arbitrary units corresponded to ≈33 basal cells.
Results
Differential S-Phase Responses and Accumulation of Cells in G2 in Xpc−/−.
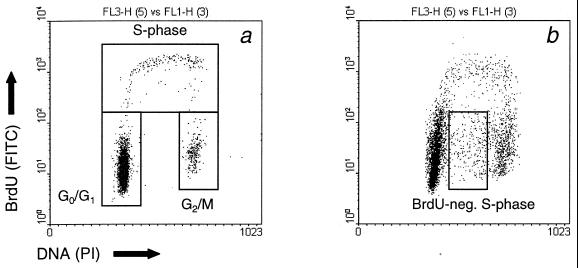
The percentage of keratinocytes in the different phases of the cell cycle was determined by simultaneous BrdUrd/DNA flow-cytometric analysis. In nonirradiated animals, no differences in distribution of cells over the different phases of the cell cycle were observed between WT, heterozygous, and homozygous mutant mice. A representative distribution is shown in Fig. 1a with ≈93% of the cells in G1/G0 phase, 3% in G2/M phase, and 3% in S phase. This distribution is the same as reported for SKH-1 hairless mice (21).
Figure 1.
Bivariate dot plots showing the distribution of the green fluorescence of the FITC anti-BrdUrd staining (DNA synthesis; y axis) versus the red fluorescence of the propidium iodide staining (DNA content; x axis) of keratinocytes from Xpc−/− mice. Mice were mock treated (a) or exposed to a single dose of UVB (b; 500 J/m2) 72 h before cell isolation. Cells were labeled in vivo with BrdUrd during the last hour before epidermal cell isolation.
Exposure of mice to UVB resulted in a change in the distribution of cells. Because no differences were observed between WT and heterozygous mutant mice after UVB irradiation, we combined the data from heterozygous mutant and WT mice in our calculations.
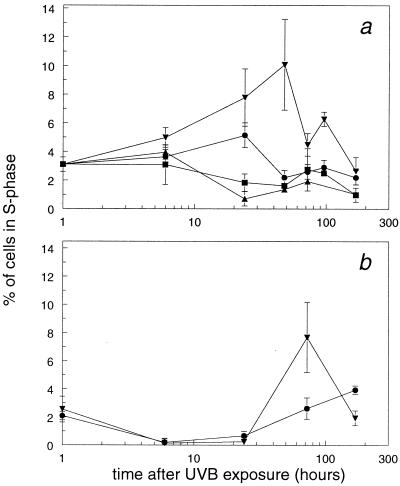
Fig. 2 shows the percentage of cells in the S phase of the cell cycle, determined as BrdUrd-positive cells as a function of time. In nonirradiated animals of all tested genotypes, about 3% of epidermal cells were S-phase cells. A UVB dose of 250 J/m2 had profound effects on the percentage of S-phase cells in the different NER-deficient and WT mice. At this dose, the percentage of S-phase cells in Xpc−/− and WT animals rose, but in both Xpa−/− and Csb−/− mice, the percentage of S-phase cells decreased (Fig. 2a). This reduction started between 6 and 24 h after UVB exposure and lasted up to 7 days after irradiation. Increasing the UVB dose to 2,000 J/m2 also evoked a transient decrease in the percentage of S-phase cells in WT and Xpc−/− mice (Fig. 2b). Exposure to a UVB dose of 40 J/m2 increased the percentage of S-phase cells in all animals tested (not shown).
Figure 2.
Mice were exposed to 250 (a) or 2,000 (b) J/m2 UVB, and epidermal cells were isolated at various times after UVB exposure. One hour before cell isolation, mice received a single dose of BrdUrd to identify S-phase cells. The percentage of cells in the S phase of the cell cycle from WT (●), Xpa−/− (▴), Xpc−/− (▾), and Csb−/− (■) mice was determined. Data are means ± SEM (n ≥ 3) or variation (n = 2).
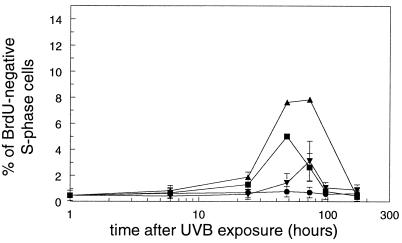
Unexpectedly, irradiated mice also contained keratinocytes that had a DNA content similar to S-phase cells, but no incorporated BrdUrd (Fig. 1b). These BrdUrd-negative S-phase cells were found in all three types of NER-deficient mice at a dose of 250 J/m2 (Fig. 3). The percentages reached maximum values of ≈8% and 3% for Xpa−/− and Xpc−/− mice, respectively, at 72 h after UVB exposure and ≈5% for Csb−/− at 48 h after UVB exposure (Fig. 3). BrdUrd-negative S phase cells were also found in Xpa−/− mice exposed to a dose of 40 J/m2 (2.9% ± 0.6% at 72 h) and in Xpc−/− mice exposed to 500 and 2,000 J/m2 (8.2% ± 2.9% and 7.9% ± 4.2 at 72 h, respectively). These cells were not found in irradiated WT mice.
Figure 3.
Mice were exposed to 250 J/m2 UVB and epidermal cells were isolated at various times after UVB exposure. One hour before cell isolation, mice received a single dose of BrdUrd to identify S-phase cells, and the percentage of BrdUrd-negative S-phase cells from WT (●), Xpa−/− (▴), Xpc−/− (▾), and Csb−/− (■) mice was determined. Data are means ± SEM (n ≥ 3) or variation (n = 2).
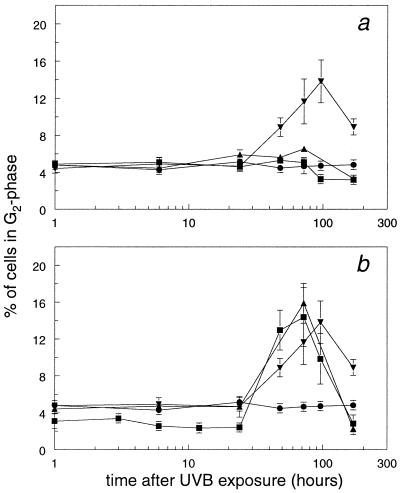
Fig. 4 shows the percentage of cells in the G2 phase of the cell cycle at various times after UVB irradiation. After exposure of mice to 250 J/m2 UVB, the percentage of G2 cells in the epidermis of Xpc−/− mice clearly increased. No such enhancement was observed in any of the other irradiated mice. The percentage of cells in G2 rose from 4.7% ± 0.2% at 24 h after UVB irradiation to a maximum of 13.3% ± 2.3% at 96 h (Fig. 4a), after which the percentage of cells in G2 decreased. The increase in the percentage of G2 cells was also observed in Xpc−/− mice after UVB doses of 500 and 2,000 J/m2, reaching maximum levels of 14.4% ± 3.2% and 15.9% ± 2.2%, respectively, at 72 h after UVB exposure (Fig. 4b). For ethical reasons, Xpa−/− and Csb−/− mice could not be exposed to these higher UVB doses. No accumulation of cells in G2 was observed in any of the NER-deficient and WT animals exposed to 40 J/m2 (data not shown).
Figure 4.
Mice were exposed to UVB, keratinocytes were isolated at various times after exposure, and the percentage of cells in the different phases of the cell cycle was determined. (a) Percentage of cells in G2 derived from WT (●), Xpa−/− (▴), Xpc−/− (▾), and Csb−/− (■) mice after a UVB dose of 250 J/m2. (b) Percentage of cells in G2 derived from Xpc−/− mice after exposure to UVB doses of 250 J/m2 (▾), 500 J/m2 (■), and 2,000 J/m2 (▴) and from WT mice exposed to 250 J/m2 (●). Data are means ± SEM (n ≥ 3) or variation (n = 2).
Enhanced Apoptosis in TCR-Defective Mice.
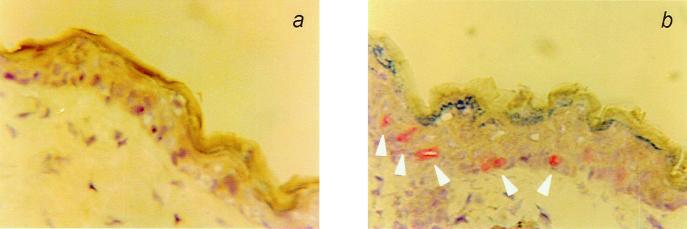
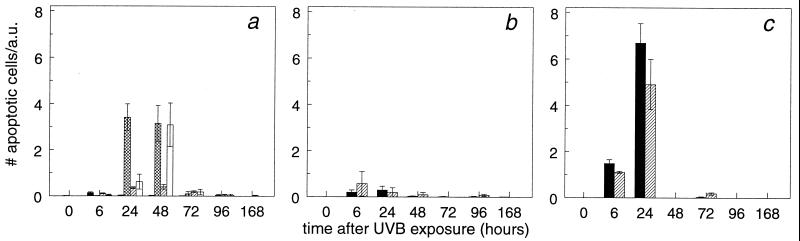
Slices (5 μm) of skin sections were stained by using anti-active caspase 3 antibodies (Fig. 5). Caspase 3 is a protein that becomes activated through cleavage of its polypeptide chain during apoptosis in a wide variety of cells. The number of active caspase 3-positive cells per arbitrary unit, considered to be sunburn cells, was taken as a measure for apoptosis. At a dose of 40 J/m2, no significant increase in apoptotic keratinocytes was observed in the NER-deficient and WT mice compared with nonirradiated mice (not shown). Fig. 6a shows the relative number of apoptotic cells in the epidermis of mice exposed to a dose of 250 J/m2 UVB. A marked increase in the number of apoptotic epidermal cells was observed in Xpa−/− and Csb−/− mice compared with WT mice at 24 and 48 h after UVB exposure. This effect was much less pronounced in Xpc−/− mice. No significant increase in the number of apoptotic cells was observed in skin of WT and Xpc−/− mice after exposure to 500 J/m2 UVB (Fig. 6b). At a dose of 2,000 J/m2 UVB, WT mice and Xpc−/− mice showed an equal increase in apoptotic cells (Fig. 6c). The data indicate that TCR protects against UVB-induced apoptosis.
Figure 5.
Immunohistochemical staining of a 5-μm section of mouse skin with an anti-active caspase 3 antibody. (a) Skin from a Xpc−/− mock-treated mouse. (b) Skin from a Xpc−/− mouse 6 h after exposure to a single dose of UVB (2,000 J/m2). Apoptotic cells are indicated with arrowheads.
Figure 6.
Mice were exposed to a single dose of 250 (a), 500 (b), or 2,000 (c) J/m2 UVB. At various times after irradiation, WT (closed bars), Xpa−/− (crosshatched bars), Xpc−/− (hatched bars), and Csb−/− (open bars) mice were killed. Sections (5 μm) were made from skin biopsies and the number of caspase 3-positive epidermal cells per arbitrary unit (a.u.) was counted. Data are means ± SEM (n ≥ 3) or variation (n = 2).
Enhanced Epidermal Hyperplasia in TCR-Defective Mice.
For determination of hyperplasia, the same sections were used as for the quantification of apoptosis. Hyperplasia was defined as an increase in the number of epidermal cell layers after UVB exposure compared with the nonirradiated epidermis. The epidermis of nonirradiated WT and NER-deficient mice consists of two cell layers. Xpa−/− and Csb−/− mice that were exposed to 40 J/m2 UVB showed a transient increase in the number of epidermal cell layers starting 48 h after irradiation (Table 1). Exposure of these mice to 250 J/m2 resulted in clear hyperplasia in Xpa−/− and Csb−/− mice, but not in WT and Xpc−/− mice. Exposure to 500 and 2,000 J/m2 resulted in hyperplasia in both WT and Xpc−/− mice (not shown).
Table 1.
UVB-induced epidermal hyperplasia in NER-deficient and WT mice
| Timer, h | No. of cell layers
|
|||||
|---|---|---|---|---|---|---|
| 40 J/m2
|
250
J/m2
|
|||||
| Xpa−/− | Csb−/− | WT | Xpa−/− | Xpc−/− | Csb−/− | |
| 0 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.05 | 2.2 ± 0.02 | 2.2 ± 0.1 | 2.2 ± 0.03 |
| 6 | 2.6 ± 0.2 | 2.1 ± 0.1 | 2.2 ± 0.05 | 2.2 ± 0.05 | 2.1 ± 0.03 | 2.2 ± 0.1 |
| 24 | 2.2 ± 0.1 | 2.5 ± 0.1 | 2.2 ± 0.03 | 2.2 ± 0.04 | 2.2 ± 0.05 | 2.2 ± 0.1 |
| 48 | 3.8 ± 0.2 | 3.1 ± 0.3 | 2.2 ± 0.04 | 3.2 ± 0.6 | 2.1 ± 0.02 | 2.2 ± 0.1 |
| 72 | 6.7 ± 1.0 | 3.9 ± 0.9 | 2.3 ± 0.04 | 5.2 ± 0.5 | 2.3 ± 0.1 | 4.4 ± 0.4 |
| 96 | 5.1 ± 1.7 | 3.9 ± 0.9 | 2.2 ± 0.4 | 4.9 ± 0.06 | 2.2 ± 0.1 | 5.0 ± 0.7 |
| 168 | 3.2 ± 0.5 | 2.7 ± 0.2 | 2.1 ± 0.04 | 3.1 ± 0.3 | 2.6 ± 0.4 | 5.6 ± 0.7 |
Hyperplasia is given as the number of epidermal cell layers at the indicated times after in vivo exposure to UVB. Data are means ±SEM (n ≥ 3) or variation (n = 2).
Discussion
TCR Facilitates Cell-Cycle Progression of UVB-Irradiated Epidermal Cells.
The experiments performed in this study show that UVB-irradiated Xpc−/− mice exhibit an increase in the percentage of epidermal cells in the G2 phase of the cell cycle. This increase did not occur immediately after irradiation, but cells started to accumulate in G2 24 h after irradiation. Remarkably, this accumulation was not observed in WT, Xpa−/−, or Csb−/− mice. Xpc−/− mice differ from Xpa−/− and Csb−/− mice in being proficient in TCR. Cultured human XPC cells are able to recover UV-inhibited RNA synthesis (23–25), whereas XPA and CSB cells are not (23, 24). Ongoing RNA synthesis appears to be a prerequisite for DNA synthesis because RNA synthesis precedes restoration of DNA synthesis in UV-irradiated normal human and XPC cells (24). It is, therefore, conceivable that TCR enables replication of damaged DNA and progression of cells through the S phase. Thus, Xpc−/− cells will replicate their DNA and reach G2, whereas Xpa−/− and Csb−/− cells lacking RNA synthesis recovery are unable to resume DNA replication after exposure to UVB doses as low as 250 J/m2 and the damaged cells will not reach the G2 phase.
As mentioned above, WT cells do not exhibit a G2 arrest, whereas Xpc−/− cells show a pronounced G2 block. To explain this distinct difference between WT and Xpc−/− mice, we hypothesize that the observed G2 arrest of cells of Xpc−/− mice is caused by replication of damaged DNA strands. UVB-irradiated Xpc−/− mice provide the unique situation that DNA replication occurs on DNA templates that are virtually not repaired and thus contain high numbers of DNA photolesions. Hence, Xpc−/− cells in the G2 phase will contain DNA harboring persistent damage. One possibility is that the signal that keeps the cells in G2 stems from persisting DNA damage itself or incorrect bases or gaps opposite a damaged DNA base (compound lesions). The compound lesions might be recognized by the mismatch repair system, giving rise to futile cycles of breakage and resynthesis. Indeed, UV-induced G2 arrest is abolished in embryonic stem cells deficient in the mismatch repair protein MSH2 (N. de Wind, personal communication). In contrast to Xpc−/− mice, WT mice are capable of removing lesions from the bulk of the DNA by GGR, thereby abolishing the signals giving rise to a G2 arrest.
The predominant lesions induced by UVB light are CPD and 6–4PP. In human WT cells, both 6–4PP and CPD are efficiently removed from the bulk of the DNA by GGR. However, rodent cells show effective GGR of 6–4PP only, thus displaying a human XPC-like phenotype as far as the removal of CPD is concerned (26). More specifically, Ruven et al. (27) showed that in the epidermis of hairless WT mice, UVB-induced CPD are effectively removed from the transcribed strand of active genes, but not from the rest of the DNA (27). These data indicate that the UVB-induced lesions that give rise to a G2 arrest in Xpc−/− mice are 6–4PP. Although it is conceivable that the extent of G2 arrest depends on the UVB dose, we did not observe significant differences in the percentage of cells in the G2 phase of UVB-irradiated Xpc−/− mice at UVB doses that vary between 250 and 2,000 J/m2. Because no G2 arrest was observed after exposure to 40 J/m2 UVB, G2 arrest is likely to start and reach maximal level at UVB doses between 40 and 250 J/m2. In this dose range, the extent of the G2 arrest is presumably dose dependent. The lack of a dose dependency at higher UV doses may be caused by saturation of the number of cells that can accumulate in G2.
The arrest of epidermal cells from Xpc−/− mice in G2 after UVB exposure is transient. The percentage of Xpc−/− cells in G2 starts to decline between 72 and 96 h after UVB exposure. At these time points, hardly any apoptosis could be observed in the epidermis of Xpc−/− mice exposed to a UVB dose of 500 J/m2, indicating that the disappearance of the cells in G2 phase is not caused by apoptosis. Although it is possible that necrosis is responsible for the removal of G2-arrested cells, several other processes could account for the disappearance of G2-arrested Xpc−/− cells. Particularly, compound lesions—i.e., incorrect bases or gaps opposite a lesion—might be recognized and repaired by the mismatch repair system. Although the majority of DNA lesions requires XPC protein for DNA repair, some lesions such as pyrimidine dimers in DNA bubbles are excised in the absence of XPC (28, 29). Wang et al. (30) showed that the mismatch repair protein heterodimer MSH2–MSH6 binds photoproduct/base mismatches in vitro. Recognition of these moieties by MSH2–MSH6 heterodimers facilitates lesion recognition and possibly repair independently of the XPC protein. Importantly, repair of lesions opposite an incorrect base introduces mutations in the DNA. Alternatively, the mismatch repair system might insert the correct base opposite photolesions. Both repair of lesions and insertion of correct bases opposite lesions will remove the signal for G2 arrest. In WT mice, UVB induces expression of MSH2 in the epidermis, indicating that MSH2 indeed may play a role in the removal of UVB-induced lesions (31).
On irradiation with 250 J/m2 UVB, epidermal cells from WT and Xpc−/− mice entered the S phase, whereas cells from Xpa−/− and Csb−/− mice did not. Only a UVB dose as high as 2,000 J/m2 blocked epidermal cell cycle progression in WT and Xpc−/− mice. The ability to enter S phase coincided with the absence of apoptosis. However, at present we do not know whether escape from apoptosis allows cells to enter S phase or that cells that do not enter S phase become apoptotic.
Surprisingly, we recovered cells from UVB-irradiated Xpc−/−, Xpa−/−, and Csb−/− mice that had a DNA content of S phase cells, but did not incorporate BrdUrd. These cells appeared ≈24 h after UVB exposure, a time point that does not coincide with the time point that apoptotic cells appear. Therefore, it is unlikely that this cell population consists of G2-phase cells undergoing apoptosis. We suggest that the BrdUrd-negative S-phase cells are cells with damaged DNA that were capable of initiating replication, but are stopped in S phase, because the replication machinery failed to bypass the lesions. The effect appears to be dose dependent. Recently, Chang et al. (32) made a similar observation after exposure of human carcinoma cells to UV.
Functional TCR Counteracts Apoptosis.
Keratinocytes from Xpa−/− and Csb−/− mice exposed to a dose of 250 J/m2 in vivo were clearly more sensitive to UVB-induced apoptosis than cells from WT and Xpc−/− mice, demonstrating that TCR protects against UVB-induced apoptosis. Similar observations were made in UVB-irradiated Xpa−/− mice and chronically UVB-exposed Xpc−/− mice by Okamoto et al. (33) and Ananthaswamy et al. (34), respectively. The protective effect of TCR is most likely caused by elimination of the apoptotic signal. It is conceivable that this signal is generated from stalled transcripts—i.e., RNA polymerase II blocked at the site of a lesion—or from the inhibition of transcription.
The difference in apoptotic response between Xpa−/− and Csb−/− cells on one hand and the Xpc−/− and WT mice on the other hand may be reflected by their p53 status. Yamaizumi et al. (35) and Ljungman et al. (36) showed that cultured human XPA and CSB fibroblasts accumulate p53 after exposure to UV at much lower doses compared with WT and XPC cells. Consequently, the difference in apoptotic response is most likely related to different levels of p53 stabilization. Ziegler et al. (37) already showed that UV-induced apoptosis of keratinocytes was completely eliminated in p53-deficient mice, indicating that UV-induced apoptosis is p53 dependent. p53 is necessary for transcription of a number of genes, including Fas (38–40). Interaction of Fas with its ligand FasL is important for UVB-induced apoptosis (41). Therefore, enhanced p53 expression in Xpa−/− and Csb−/− mice might enhance Fas expression and facilitate apoptosis.
TCR Suppresses Induction of Hyperplasia After UVB Irradiation.
Apart from apoptosis and modulation of cell cycle progression, UVB induces another alteration in the mouse skin, namely hyperplasia. Like sensitivity to UVB-induced apoptosis and sensitivity to sunburn, the sensitivity of UVB-irradiated mice to hyperplasia appears to correlate with the absence of TCR. Once exposed to equal UVB doses, Xpa−/− and Csb−/− mice are more prone to hyperplasia compared with WT and Xpc−/− mice. Although we did not notice a marked difference in hyperplasia between Xpc−/− and WT mice, Ananthaswamy et al. (34) did observe a difference in chronically exposed Xpc−/− mice. A UVB dose as low as 40 J/m2 evoked a hyperplastic response in the skin of Xpa−/− and Csb−/− mice, but no apoptosis. Therefore, it is unlikely that hyperplasia is a consequence of apoptosis.
In summary, we investigated the relationship between NER, or rather certain NER defects, and acute cellular responses in the epidermis of UVB-irradiated NER-deficient mice. It was found previously that active TCR protects against UVB-induced acute effects—e.g., edema and erythema (19, 20)—but not against skin cancer (17, 20). We found that (i) TCR protects against UVB-induced apoptosis (sunburn cells); (ii) the occurrence of a late G2 arrest in keratinocytes of XPC−/− mice appears to be the result of a replication of damaged DNA; and (iii) TCR prevents hyperplasia after a single UVB exposure.
Acknowledgments
We thank Dr. A. Bloem for support in operating the fluorescence-activated cell sorter and H. Sturkenboom for his biotechnical support. This work was supported by Dutch Cancer Society Grant UU 97–1531 and CA-44247 from the U.S. Public Health Service (E.C.F.).
Abbreviations
- NER
nucleotide excision repair
- GGR
global genome repair
- TCR
transcription-coupled repair
- XP
xeroderma pigmentosum
- XPA
XP group A
- XPC
XP group C
- CS
Cockayne syndrome
- CSB
CS group B
- WT
wild-type
- CPD
cyclobutane pyrimidine dimer
- 6–4PP
pyrimidine(6–4)pyrimidone photoproducts
- RT
room temperature
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200226697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200226697
References
- 1.Bohr V A, Smit C A, Okumoto D S, Hanawalt P C. Cell. 1985;40:359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- 2.Mellon I, Spivak G, Hanawalt P C. Cell. 1987;51:241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- 3.Cleaver J E, Kraemer K H. In: The Metabolic Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1989. pp. 2949–2971. [Google Scholar]
- 4.Bootsma D. Eur J Cancer. 1993;29A:1482–1488. doi: 10.1016/0959-8049(93)90026-c. [DOI] [PubMed] [Google Scholar]
- 5.Kraemer K H, Lee M M, Scotto J. Carcinogenesis. 1984;5:511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 6.Kraemer K H, Myung M L, Scotto J. Arch Dermatol. 1987;123:241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- 7.Ma L, Hoeijmakers J H J, van der Eb A J. Biochim Biophys Acta. 1995;1242:137–164. doi: 10.1016/0304-419x(95)00008-4. [DOI] [PubMed] [Google Scholar]
- 8.Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. Microbiol.; 1995. [Google Scholar]
- 9.Venema J, van Hoffen A, Karcagi V, Natarajan A T, van Zeeland A A, Mullenders L H. Mol Cell Biol. 1991;11:4128–4134. doi: 10.1128/mcb.11.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venema J, Mullenders L H F, Natarajan A T, van Zeeland A A, Mayne L V. Proc Natl Acad Sci USA. 1990;87:4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Hoffen A, Natarajan A T, Mayne L V, van Zeeland A A, Mullenders L H F, Venema J. Nucleic Acids Res. 1993;21:5890–5895. doi: 10.1093/nar/21.25.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries A, van Oostrom C T, Hofhuis F M, Dortant P M, Berg R J, de Gruijl F R, Wester P W, Van Kreijl C F, Capel P J, van Steeg H. Nature (London) 1995;377:169–173. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- 13.Nakane H, Takeuchi S, Yuba S, Saijo M, Nakatsu Y, Murai H, Nakatsuru Y, Ishikawa T, Hirota S, Kitamura Y. Nature (London) 1995;377:165–168. doi: 10.1038/377165a0. [DOI] [PubMed] [Google Scholar]
- 14.Cheo D L, Ruven H J T, Meira L B, Hammer R E, Burns D K, Tappe N J, van Zeeland A A, Mullenders L H F, Friedberg E C. Mutat Res. 1997;374:1–9. doi: 10.1016/s0027-5107(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 15.Berg R J, Ruven H J, Sands A T, de Gruijl F R, Mullenders L H. J Invest Dermatol. 1998;110:405–409. doi: 10.1111/j.1523-1747.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Horst G T, van Steeg H, Berg R J, van Gool A J, de Wit J, Weeda G, Morreau H, Beems R B, Van Kreijl C F, de Gruijl F R, et al. Cell. 1997;89:425–435. doi: 10.1016/s0092-8674(00)80223-8. [DOI] [PubMed] [Google Scholar]
- 17.Sands A T, Abuin A, Sanchez A, Conti C J, Bradley A. Nature (London) 1995;377:162–165. doi: 10.1038/377162a0. [DOI] [PubMed] [Google Scholar]
- 18.Cheo D L, Meira L B, Hammer D E, Burns D K, Doughty A T B, Friedberg E C. Curr Biol. 1996;6:1691–1694. doi: 10.1016/s0960-9822(02)70794-x. [DOI] [PubMed] [Google Scholar]
- 19.Berg R J W, de Vries A, van Steeg H, de Gruijl F R. Cancer Res. 1997;57:581–584. [PubMed] [Google Scholar]
- 20.Berg R J W, Heggert Rebel H, van der Horst G T J, van Kranen H J, Mullenders L H F, van Vloten W A, de Gruijl F R. Cancer Res. 2000;60:2858–2863. [PubMed] [Google Scholar]
- 21.de Laat A, Kroon E D, de Gruijl F R. Photochem Photobiol. 1997;65:730–735. doi: 10.1111/j.1751-1097.1997.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 22.de Laat A, van Tilburg M, van der Leun J C, van Vloten W A, de Gruijl F R. Photochem Photobiol. 1996;63:492–497. doi: 10.1111/j.1751-1097.1996.tb03075.x. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann A R, Kirk-Bell S, Mayne L. Cancer Res. 1979;39:4237–4241. [PubMed] [Google Scholar]
- 24.Mayne L V, Lehmann A R. Cancer Res. 1982;42:1473–1478. [PubMed] [Google Scholar]
- 25.van Hoffen A, Kalle W H, de Jong-Versteeg A, Lehmann A R, van Zeeland A A, Mullenders L H. Nucleic Acids Res. 1999;27:2898–2904. doi: 10.1093/nar/27.14.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Zeeland A A, Smith C A, Hanawalt P C. Mutat Res. 1981;82:173–189. doi: 10.1016/0027-5107(81)90148-2. [DOI] [PubMed] [Google Scholar]
- 27.Ruven H T J, Seelen C M J, Lohman P H M, van Kranen H, van Zeeland A A, Mullenders L H F. Oncogene. 1994;9:3427–3432. [PubMed] [Google Scholar]
- 28.Mu D, Hsu D, Sancar A. J Biol Chem. 1996;271:8285–8294. doi: 10.1074/jbc.271.14.8285. [DOI] [PubMed] [Google Scholar]
- 29.Mu D, Sancar A. J Biol Chem. 1997;272:7570–7573. doi: 10.1074/jbc.272.12.7570. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Lawrence C W, Li G M, Hays J B. J Biol Chem. 1999;274:16894–16900. doi: 10.1074/jbc.274.24.16894. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y-P, Lou Y-R, Yen P, Mitchell D, Huang M-T, Conney A H. Cancer Res. 1999;59:4591–4602. [PubMed] [Google Scholar]
- 32.Chang D, Chen F, Zhang F, McKay B C, Ljungman M. Cell Growth Differ. 1999;10:155–162. [PubMed] [Google Scholar]
- 33.Okamoto H, Mizuno K, Itoh T, Tanaka K, Horio T. J Invest Dermatol. 1999;113:802–807. doi: 10.1046/j.1523-1747.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 34.Ananthaswamy H N, Ouhtit A, Evans R L, Gorny A, Khaskina P, Sands A T, Conti C J. Oncogene. 1999;18:7395–7398. doi: 10.1038/sj.onc.1203147. [DOI] [PubMed] [Google Scholar]
- 35.Yamaizumi M, Sugano T. Oncogene. 1994;9:2775–2784. [PubMed] [Google Scholar]
- 36.Ljungman M, Zhang F. Oncogene. 1996;13:823–831. [PubMed] [Google Scholar]
- 37.Ziegler A, Jonason A S, Leffel D J, Simon J A, Sharma H W, Kimmelman J, Remington L, Jacks T, Brash D E. Nature (London) 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 38.Galle P R, Hofmann W J, Walczak H, Schaller H, Otto G, Stremmel W, Krammer P H, Runkel L. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller M, Strand S, Hug H, Heinemann E-M, Walczak H, Hofmann W J, Stremmel W, Krammer P H, Galle P R. J Clin Invest. 1997;99:403–413. doi: 10.1172/JCI119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman S L, Galle P R, Stremmel W, Oren M, et al. J Exp Med. 1998;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill L L, Ouhtit A, Loughlin S M, Kripke M L, Ananthaswamy H N, Owen-Schaub L B. Science. 1999;285:898–900. doi: 10.1126/science.285.5429.898. [DOI] [PubMed] [Google Scholar]