Abstract
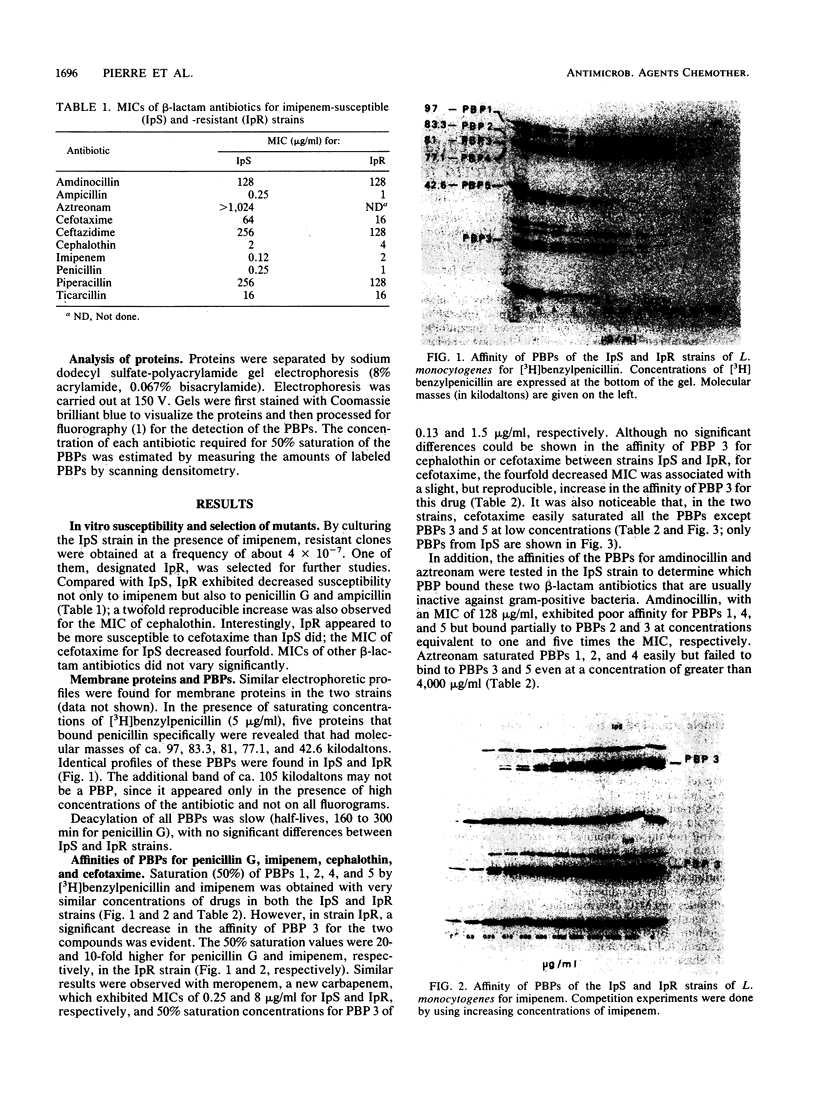
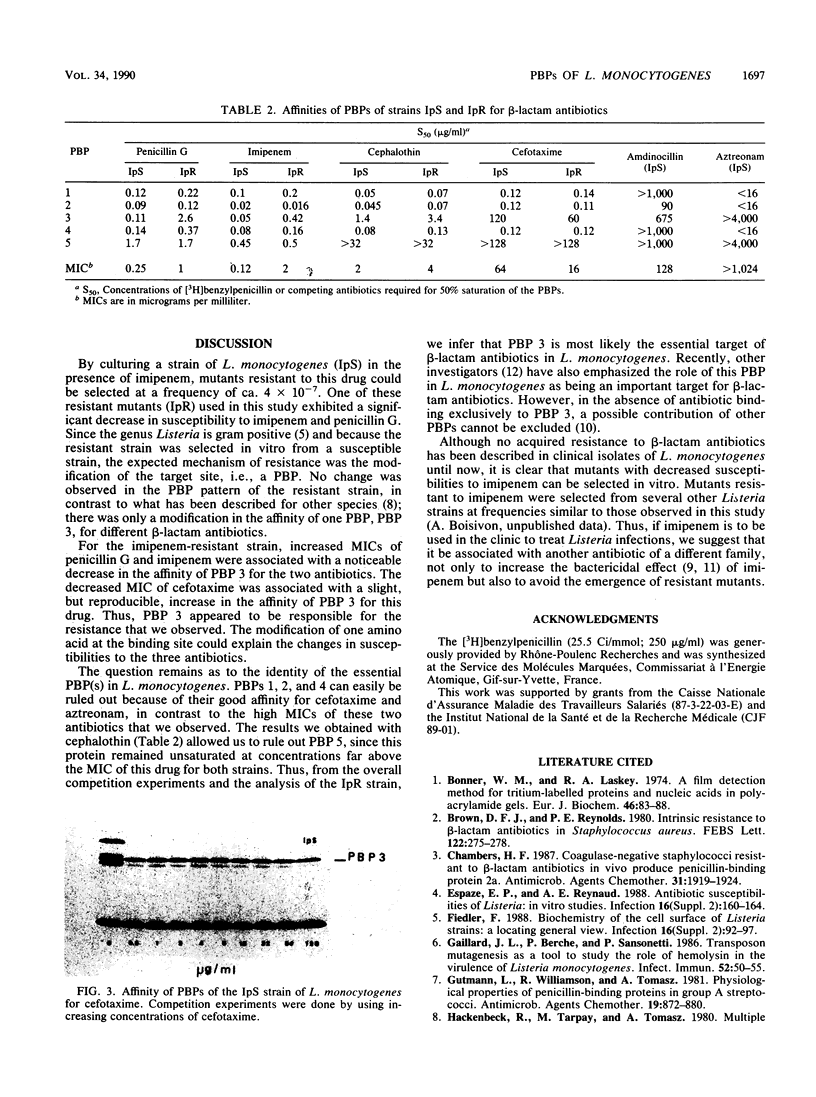
A mutant with decreased susceptibility to imipenem (IpR) was selected in vitro from a susceptible clinical isolate of Listeria monocytogenes (IpS). IpR exhibited decreased susceptibility to penicillin G (4 x MIC) and imipenem (16 x MIC) and increased susceptibility to cefotaxime (0.25 x MIC). Electrophoretic profiles of membrane proteins and penicillin-binding proteins (PBPs) were identical in the two strains; each strain had five PBPs with molecular masses of ca. 97, 83.3, 81, 77.1, and 42.6 kilodaltons. A decreased affinity of PBP 3 for penicillin G and imipenem (10-fold) was observed in IpR. In contrast, the affinity of PBP 3 for cefotaxime in IpR was increased twofold and correlated with the decreased MIC of this drug. From these findings and competition experiments with different beta-lactam antibiotics, we conclude that the alteration of PBP 3 is responsible for the decreased susceptibility of IpR to penicillin and imipenem and that PBP 3 might be an essential target of beta-lactam antibiotics in L. monocytogenes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Chambers H. F. Coagulase-negative staphylococci resistant to beta-lactam antibiotics in vivo produce penicillin-binding protein 2a. Antimicrob Agents Chemother. 1987 Dec;31(12):1919–1924. doi: 10.1128/aac.31.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Tomasz A. Physiological properties of penicillin-binding proteins in group A streptococci. Antimicrob Agents Chemother. 1981 May;19(5):872–880. doi: 10.1128/aac.19.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Medoff G., Leech I., Wennersten C., Kunz L. J. Antibiotic synergism against Listeria monocytogenes. Antimicrob Agents Chemother. 1972 Jan;1(1):30–34. doi: 10.1128/aac.1.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H. Perinatal listeriosis. Tolerance of a clinical isolate of Listeria monocytogenes for ampicillin and resistance against cefotaxime. Chemotherapy. 1981;27(6):423–431. doi: 10.1159/000238012. [DOI] [PubMed] [Google Scholar]
- Vicente M. F., Pérez-Dáz J. C., Baquero F., Angel de Pedro M., Berenguer J. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for beta-lactams. Antimicrob Agents Chemother. 1990 Apr;34(4):539–542. doi: 10.1128/aac.34.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]