Abstract
Cyclooxygenase-2 (COX-2) is up-regulated in many cancers and is a rate-limiting step in colon carcinogenesis. Nonsteroidal antiinflammatory drugs, which inhibit COX-2, prevent colon cancer and cause apoptosis. The mechanism for this response is not clear, but it might result from an accumulation of the substrate, arachidonic acid, an absence of a prostaglandin product, or diversion of the substrate into another pathway. We found that colon adenocarcinomas overexpress another arachidonic acid-utilizing enzyme, fatty acid-CoA ligase (FACL) 4, in addition to COX-2. Exogenous arachidonic acid caused apoptosis in colon cancer and other cell lines, as did triacsin C, a FACL inhibitor. In addition, indomethacin and sulindac significantly enhanced the apoptosis-inducing effect of triacsin C. These findings suggested that unesterified arachidonic acid in cells is a signal for induction of apoptosis. To test this hypothesis, we engineered cells with inducible overexpression of COX-2 and FACL4 as “sinks” for unesterified arachidonic acid. Activation of the enzymatic sinks blocked apoptosis, and the reduction of cell death was inversely correlated with the cellular level of arachidonic acid. Inhibition of the COX-2 component by nonsteroidal antiinflammatory drugs restored the apoptotic response. Cell death caused by exposure to tumor necrosis factor α or to calcium ionophore also was prevented by removal of unesterified arachidonic acid. We conclude that the cellular level of unesterified arachidonic acid is a general mechanism by which apoptosis is regulated and that COX-2 and FACL4 promote carcinogenesis by lowering this level.
Arachidonic acid (AA; 20:4, n-6) is an essential polyunsaturated fatty acid, and the oxidation of AA generates prostaglandins and leukotrienes. Accumulating evidence indicates that AA metabolism plays an important role in carcinogenesis (1–3). For example, cyclooxygenase 2 (COX-2), the inducible isoform of the enzyme that catalyzes the committed step in prostaglandin synthesis from AA, was shown to be an essential step in colon carcinogenesis. Nonsteroidal antiinflammatory drugs (NSAIDs) lower colon cancer risk in population studies (4) and prevent carcinogen-induced cancers in animals (5, 6). Genetic studies showed that COX-2 plays a crucial role in the genesis of polyps (7). Moreover, COX-2 is up-regulated in colon cancer (8, 9), as is the reticulocyte-type 15-lipoxygenase (LOX) (10). These findings suggest that multiple pathways of AA metabolism are activated during carcinogenesis. A recent report showed that drug-treated tumors show an increase in the level of polyunsaturated fatty acids (11).
Several lines of evidence indicate that the level of unesterified AA in cells can serve as a second messenger (12) and regulate apoptosis. One mutant line, in which the cytosolic phospholipase A2 (cPLA2) activity was significantly reduced, was shown to be resistant to tumor necrosis factor (TNF) α-mediated cell killing. This suggests that blocking endogenous AA release renders cells resistant to TNF-α killing (13). Along the same line, it has been shown that suppression of cPLA2 activity led to a decrease in cell death, whereas overexpression of cPLA2 enhanced cell death (13, 14). Moreover, a defect in the rate-limiting enzyme for AA biosynthesis, Δ6-desaturase, has been reported in a cell line selected by virtue of being resistant to TNF-α killing (15). One interpretation for the above results is that the reduced cellular AA pool sets a limitation on the level of endogenous AA that can accumulate in response to TNF-α, which confers resistance to cell death. However, this interpretation does not distinguish between an effect of unesterified AA itself or a downstream product, i.e., an eicosanoid. It has been reported that inhibitors of 12-LOX induced apoptosis (16). Likewise, NSAIDs gave a similar response in the colon cancer lines, and the apoptosis-inducing effect could be mimicked by the addition of AA (17). In addition, Chilton and his colleagues (18, 19) reported that blocking arachidonate–phospholipid remodeling by inhibitors of CoA-independent transacylase induced apoptosis, which correlated with an accumulation of intracellular unesterified AA.
In this study, we found that fatty acid-CoA ligase (FACL) 4, which highly prefers AA as substrate (20), also was up-regulated in colon adenocarcinomas. This suggests that the alterations of AA metabolic pathways lower the level of unesterified AA and thereby prevent apoptosis. To test this hypothesis, we established human epithelial lines with stable, inducible expression of FACL4 and/or COX-2, allowing us to answer whether AA itself is the signal for apoptosis; if so, activating pathways that consume AA should protect against apoptosis even though they yield different products.
Materials and Methods
Tumor Tissues and Reverse Transcription–PCR.
Total RNA was prepared from surgically removed colon adenocarcinoma and the adjacent normal tissues from the same patient. Colon cancer cell lines were from American Type Culture Collection. Reverse transcription–PCR was carried out by using primers corresponding to the coding sequence of human FACL4 or COX-2 and β-actin primer/competimer mix (Ambion). The sizes of the amplified fragments for FACL4 and COX-2 are 441 and 480 bp, and the β-actin primers amplify a fragment of 294 bp.
Cell Death Detection ELISA.
Cells were plated on 96-well plates at 1 × 104 cells per well in complete medium. AA (Sigma) was applied, and apoptotic cell death was determined after 44 h with the cell death detection ELISA (Boehringer Mannheim). AA was suspended in ethanol, aliquoted into a small volume, sealed under an inert nitrogen stream, and stored at −20°C. One aliquot was used only for one experiment, and the remaining AA was discarded to minimize autoxidation. AA was first added to a small volume of full medium, sonicated briefly, and then applied to the cultured cells.
Stable Cell Lines.
The ecdysone-inducible expression system (Invitrogen) was used to construct stable lines overexpressing human FACL4, COX-2, COX-2 mutant, and both FACL4 and COX-2. COX-2 mutant cDNA expresses a catalytically inactive COX-2 with change of a single amino acid residue (L547K). The cDNAs were cloned into pIND(SP1) (neomycin). The cDNA constructs or the empty vector pIND(SP1) was transfected into EcR293 cells, which were then selected in 400 μg/ml geneticin and 400 μg/ml zeocin. A number of single colonies were screened for overexpression of FACL4, COX-2 wild type, and COX-2 mutant by immunoblotting against anti-FACL4 (21) and anti-COX-2 antibodies (a gift from Jacques Maclouf, Hôpital Lariboisiere, Paris) and by measurement of the CoA ligase activity for FACL4 (22) or the prostaglandin (PG) E2 secretion for COX-2 wild type (ELISA, Assay Design). The double stable line was generated by stably transfecting the COX-2 stable cells with another pIND(SP1)-FACL4 cDNA construct (hygromycin). The cells were selected in 400 μg/ml geneticin, 400 μg/ml zeocin, and 50 μg/ml hygromycin B. The positive colonies were screened as described above.
Cell Survival.
Cell viability was determined with crystal violet staining (13).
In Situ Cell Death Detection.
Cells were plated on 8-well glass chamber slides precoated with poly-l-lysine. The terminal deoxynucleotidyltransferase-mediated dUTP end labeling assay was performed with the in situ cell death detection kit (Boehringer Mannheim).
Arachidonic Acid Release.
Cells were plated on 6-well plates precoated with poly-l-lysine at 8 × 105 cells per well. They were then prelabeled with [3H]AA (0.4 μCi/ml medium) (91.8 Ci/mmol, 100 μCi/ml, NEN) for 24 h. After a wash, serum-free DMEM supplemented with 1% BSA (fatty acid-free) was added. A small portion of the medium was withdrawn at time intervals, and unesterified fatty acids were extracted (23). The heptane phase was collected, and the 3H-labeled AA was determined by a scintillation counter. The medium was extracted 28 h later, and AA release was determined again. The percentage of AA release (+P/−P) was calculated by first subtracting released AA after 4 h (background) from that at 32 h in the same dish and then dividing the release level after ponasterone treatment (+P) by that of control (−P) in the same stable line. The AA release stimulated by A23187 was calculated by subtracting the released AA by unstimulated cells from that released by A23187-stimulated counterparts.
Lipid Peroxidation Assay.
Cells were plated and treated with AA for 48 h. They were then suspended in 300 μl H2O, and the cell lysates were prepared by repeatedly freezing and thawing. The cellular malondialdehyde and 4-hydroxynonenal levels were determined with the lipid peroxidation assay kit (Calbiochem).
Transfection with Bcl-2 cDNA Construct.
The cells were transfected with human Bcl-2 cDNA construct by using LipofectAMINE (Life Technologies). Two micrograms Bcl-2 cDNA and 1 μg luciferase cDNA constructs were transfected into ≈4 × 105 cells. The cells were harvested 48 h later and replated at 1 × 104 cells per well on 96-well plates. The transfectants were exposed to 300 μM AA, and apoptosis was examined 44 h later.
Indirect Fluorescent Immunostaining.
Cells were cultured in poly-l-lysine-precoated eight-chamber slides and were fixed with 3.7% paraformaldehyde. The slides were then blocked and the anti-active caspase 3 antibody (PharMingen) was applied, followed by incubation with FITC-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch).
Results
The Expression of FACL4 Is Increased in Colon Adenocarcinomas.
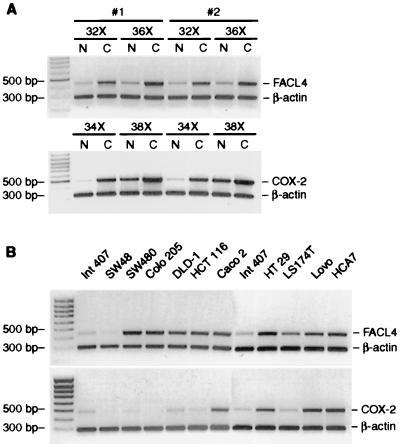
We previously cloned the human isoform 4 of the fatty acid CoA-ligase gene. It encodes an enzyme that highly prefers AA as substrate and that converts it into arachidonoyl CoA ester (20). Because FACL4 and COX-2 both use AA as substrate and COX-2 was shown to be induced in colon cancer (8, 9), we asked whether FACL4 is also up-regulated in colon adenocarcinomas. The results showed that in addition to COX-2, the expression of FACL4 was significantly increased in the colon adenocarcinoma compared with the adjacent normal tissue from the same patient. We observed a 2- to 14-fold increase in the expression of FACL4 in 23 of 24 colon adenocarcinoma samples, and a representative result from two patients is shown in Fig. 1A. Additional experiments showed an increased expression of FACL protein, examined by Western blotting and immunostaining, in the tumor samples compared with the normal tissue (not shown). We also found that many colon carcinoma cell lines express a significantly higher level of FACL4 than does the intestinal epithelial line Int 407 (Fig. 1B).
Figure 1.
The expression of FACL4 and COX-2 is increased in colon adenocarcinomas and cell lines. (A) Expression in adenocarcinomas. Quantitative reverse transcription–PCR was performed on RNAs isolated from 24 pairs of colon adenocarcinomas and the adjacent normal tissues. A representative reverse transcription–PCR quantitation from two patients is shown. Amplification with the FACL4 amplicons (upper) and COX-2 amplicons (lower). Amplification of β-actin was used for normalization. FACL4 was amplified for 32 and 36 cycles and COX-2 for 34 and 38 cycles. The identity of the 480-bp fragment amplified with the FACL4 primers was verified by sequencing, and it completely matched the cDNA sequence of human FACL4 (20). C, colon adenocarcinoma; N, normal colon tissue from the same patient. (B) Expression in colon cancer cell lines. The legends on top of the gel indicate the cell line from which RNA was extracted.
An FACL Inhibitor Induces Apoptosis and Synergizes with NSAIDs in Induction of Apoptosis.
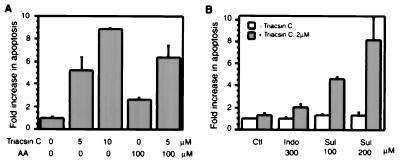
Previous reports indicated that inhibition of COX-2 by NSAIDs in transformed cells results in apoptosis. We evaluated whether triacsin C, an inhibitor of FACL, has a similar effect. The result revealed that triacsin C induced apoptosis in a concentration-dependent manner in HT 29 cells; at 10 μM it caused a 9-fold increase compared with control cells (P < 0.001) (Fig. 2A). Furthermore, exogenous AA slightly induced apoptosis at 100 μM, and cotreatment with triacsin C caused apoptotic cell death to a greater extent (P < 0.02). This finding indicates that the induction of apoptosis by triacsin C is mediated through inactivation of the FACL4 pathway and, subsequently, through an accumulation of unesterified AA in cells. We further tested whether blocking these two metabolic pathways simultaneously exerts a synergistic effect on apoptosis. We found that triacsin C at a concentration (2 μM) which otherwise did not cause cell death in HT 29 cells slightly induced apoptosis when indomethacin was added. The apoptosis-inducing effect of triacsin C was increased 4- to 8-fold in the presence of sulindac (P < 0.004) (Fig. 2B). A similar synergy was detected in human kidney epithelial 293 cells (not shown). Thus, it is likely that the signal for apoptosis is an elevated level of AA, and triacsin C and NSAIDs induce apoptosis by blocking the metabolic removal of unesterified AAs.
Figure 2.
Triacsin C induces apoptosis (A) and synergizes with NSAIDs in induction of apoptosis (B). Triacsin C and AA were added (in A), or triacsin C with either indomethacin (Indo) or sulindac (Sul) (in B) were added to HT 29 cells at the indicated concentrations. Apoptosis was determined by cell death ELISA 44 h later (A) and 72 h later (B), respectively. Ctl, cells without NSAID treatment. The values shown are the means of triplicate determinations. Error bars in this and subsequent figures represent the standard deviation.
Arachidonic Acid Induces Apoptosis in Epithelial Cells and Involves Activation of Caspase 3.
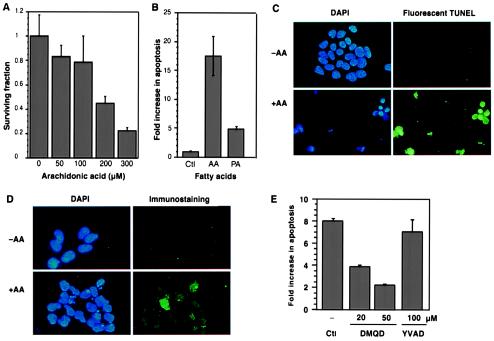
To evaluate the mechanism implicated in the up-regulation of AA-utilizing enzymes in tumor tissues, we engineered a cellular model that allowed us to manipulate the intracellular unesterified AA level. Initially, we examined the fate of 293 cells exposed to exogenous AA and found that survival fell to 23% of the control as the fatty acid concentration was increased (Fig. 3A). The portion of cell death attributable to apoptosis was estimated in an assay that detects the formation of mono- and oligonucleosomes. A high level (18-fold over the control) was detected when the cells were exposed to 300 μM AA, but no increase was observed when they were exposed to palmitic acid (Fig. 3B). The control cells had intact nuclei, whereas the cells treated with AA showed nuclear condensation and a high level of DNA strand breaks (Fig. 3C). Thus, AA induces apoptosis in a concentration-dependent manner. Moreover, we detected caspase 3-like activity in AA-treated cells in a time- and concentration-dependent manner compared with control cells—the maximal increase was ≈4.5-fold, and the peak was at 17 h (not shown). Activation of caspase 3 in individual cells was also detected, and the staining pattern revealed that the level of activated caspase 3 correlated with the extent of chromatin fragmentation (Fig. 3D). We next added inhibitors of either caspases 1 and 4 [z (benzyloxycarbonyl-YVAD-fmk (fluoromethylketone)] or caspase 3 (z-DMQD-fmk) to the cells before exposing them to exogenous AA. We found that the caspase 3 inhibitor but not the caspases 1 and 4 inhibitor significantly blocked apoptosis (P < 0.001 at both concentrations) (Fig. 3E). This indicates that activation of caspase 3 is an essential downstream event in AA-induced apoptosis.
Figure 3.
Arachidonic acid induces apoptosis via activation of caspase 3. (A) Determination of cell survival. AA was added to 293 cells at the indicated concentrations and the surviving fraction of cells was determined after 44 h. (B) Increased apoptosis in cells exposed to AA. Cells were treated with AA (300 μM) or palmitic acid (300 μM) under the same conditions as in A, and then apoptosis was detected with an ELISA. The mean values from triplicate determinations are shown (for AA, P < 0.01). In multiple experiments the concentration of AA that induced apoptosis in a substantial fraction of the cells varied between 200 and 300 μM. (C) In situ detection of apoptosis. The 293 cells were treated with AA (300 μM; +AA) or vehicle alone (−AA) for 44 h. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI), and DNA strand breaks in individual cells were detected with a fluorescent terminal deoxynucleotidyltransferase-mediated dUTP end labeling (TUNEL) assay. (Upper) Same field of cells (−AA) photographed under UV (left) and FITC filters (right) (×80). (Lower) Apoptotic cells (+AA). (D) Immunostaining with an anti-active caspase 3 antibody. (Upper) Control cells (−AA). (Lower) Cells exposed to 300 μM AA. (Left) DAPI staining; (Right), immunostaining with an antibody selective for active caspase 3 (×100). (E) Blocking of AA-induced apoptosis by inhibition of caspase 3. The 293 cells were preincubated with caspase inhibitors for 1 h at the indicated concentrations, AA (300 μM) was added, and apoptosis was determined 44 h later. DMQD [z (benzyloxycarbonyl)-DMQD-fmk (fluoromethylketone)], caspase 3 inhibitor; YVAD (z-YVAD-fmk), caspases 1 and 4 inhibitor (Peptide Institute). The values represent the means from triplicate determinations.
Activation of AA-Utilizing Pathways Prevents AA-Induced Apoptosis by Reducing the Level of Intracellular Unesterified AA.
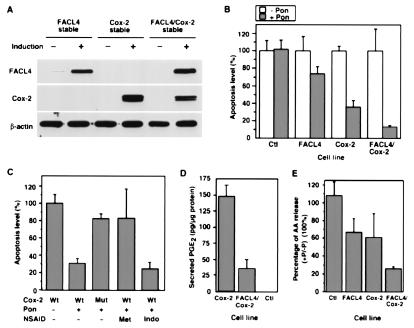
We next constructed stably transfected lines that express human FACL4, human COX-2, or both on induction. The expression level of FACL4, COX-2, or both on induction was detected in the stable lines by Western blotting (Fig. 4A). In addition, we isolated lines with inducible expression of a mutant COX-2 (L547K) that lacks catalytic activity (not shown). We then tested whether overexpression of FACL4 and/or COX-2 prevented AA-induced apoptosis. A reduced level of apoptosis was observed in cells that overexpressed FACL4 or COX-2 compared with control cells (Fig. 4B). The reduction in apoptosis was not a side effect of ponasterone, the agent used to induce expression of the enzymes, because the control cells harboring the empty vector generated similar levels of signals with or without induction. The attenuation of apoptosis was much more pronounced when cells overexpressed both FACL4 and COX-2 (Fig. 4B). In these cells, the removal of AA through the COX-2 pathway probably supersedes that through the FACL4 pathway, because the Km values with AA as substrate for COX-2 and FACL4 are 5 and 10–15 μM, respectively (24, 25). We conclude that overexpression of FACL4 or COX-2 partially prevents AA-induced apoptosis, and simultaneous overexpression of both enzymes has an additive effect.
Figure 4.
Overexpression of COX-2 or FACL4 inhibits apoptosis. (A) Conditional expression levels of FACL4 and COX-2 in stably transfected lines. Cells were treated with (+) or without (−) ponasterone (Pon, 1 μg/ml) for 48 h, and Western blotting was carried out with anti-FACL4 and anti-COX-2 antibodies, respectively. The loading was normalized with an anti-β-actin antibody. (B) Reduction of AA-induced apoptosis by overexpression of COX-2, FACL4, and both together. The 293 cells stably transfected with an empty control vector (Ctl), FACL4 cDNA, COX-2 cDNA, and both FACL4 and COX-2 cDNAs were uninduced (− Pon) or induced for overexpression (+ Pon) as in A. AA (300 μM) was added after 24 h of induction, and apoptosis was determined 44 h later. The increase in apoptosis induced by AA varied between 6- and 20-fold relative to the signal in cells without treatment. The plot is a comparison of the relative apoptosis levels among the stable lines and is representative of three independent experiments. The values shown are the averages of triplicate determinations. (C) Requirement for the catalytic activity for the COX-2 mediated prevention of apoptosis. Cells were uninduced or induced for overexpression as above. The mutant (Mut) COX-2 was expressed at a level similar to that of the wild type (Wt) as assessed by immunoblotting, but there was no increase above background in the amount of prostaglandin synthesized. COX inhibitors were administered 6 h later as indicated. AA (300 μM) was then added after 2 h, and apoptosis was determined 44 h later. The plot is a representative result from three independent experiments, in which triplicate determinations were performed. Met, 50 μM 6-methoxy-2-naphthylacetic acid, Indo, 10 μM indomethacin. (D) Reduction of prostaglandin synthesis by coexpression of FACL4 with COX-2. The COX-2, FACL4/COX-2 stable, and control cells carrying an empty vector were induced for 48 h and washed and incubated with 20 μM AA at 37°C for 30 min. The amount of PGE2 synthesized was determined by ELISA. Four additional clones of the FACL4/COX-2 double stable cells were examined, and all secreted a lower level of PGE2, ranging from 16 to 56%, than the COX-2 single stable lines. (E) The levels of unesterified AA in the stable lines. The [3H]AA released to the supernatant was determined, and the values shown are averages from multiple experiments (n = 4, P < 0.05).
We next asked whether other polyunsaturated fatty acids induced apoptosis and observed that eicosapentaenoic acid and docosahexaenoic acid were effective. Both of these fatty acids are substrates for COX-2 although docosahexaenoic acid is not as good as either AA or eicosapentaenoic acid (24). The increase in apoptosis was 8.5-, 9.1-, 15.1- and 4.2-fold after treatment with 200 μM AA, 200 μM eicosapentaenoic acid, 100 μM docosahexaenoic acid, and 200 μM palmitic acid, respectively. By inducing COX-2 overexpression, apoptosis in response to AA was reduced by 36.7% (n = 3, P < 0.001), to eicosapentaenoic acid by 34.0% (n = 3, P < 0.01), to docosahexaenoic acid by 18.5% (n = 3, P > 0.05), and to palmitic acid by only 8.1% (n = 3, P > 0.05). Thus, prevention of apoptosis by overexpression of COX-2 correlated with the suitability of the fatty acid as substrate. To test whether the protective effect of COX-2 required its catalytic activity, as suggested by the results with different fatty acids, we compared the level of apoptosis in cells with wild-type COX-2 to that in cells with an inactive mutant COX-2 (L547K). In contrast to wild-type COX-2, overexpression of the mutant COX-2 did not inhibit apoptosis (Fig. 4C). We next tested the effect of NSAIDs on this response and found that 6-methoxy-2-naphthylacetic acid (50 μM), a selective inhibitor of COX-2, abolished the prevention of apoptosis by COX-2. In contrast, indomethacin at 10 μM, a concentration that completely inhibits COX-1 but only suppresses 60% of the COX-2 activity in the COX-2 stable cells (not shown) (26), did not alter the protective response (Fig. 4C). Thus, inhibition of COX-2 had a proapoptotic effect.
The protection from apoptosis could have resulted from the extensive removal of unesterified AA or alternatively from the production of metabolites. To distinguish between these two mechanisms, we performed several experiments: first, we compared the secretion of PGE2, the major product of the COX-2 pathway, in the COX-2 single and the FACL4/COX-2 double stable lines. The double stable cells secreted ≈28% of the amount of PGE2 secreted by the COX-2 single stable cells (Fig. 4D). This difference in secretion most likely resulted from competition between COX-2- and FACL4-mediated pathways for the pool of unesterified AA. This result strongly favors the interpretation that depletion of AA and not the generation of products was responsible for the prevention, because the double stable cells prevented apoptosis more effectively than the COX-2 single stable cells even though they produced a much lower level of prostaglandins. Furthermore, we directly added PGE2 (50 μM) and PGJ2 (5 μM) to the cells and found that they did not improve cell viability in the presence of exogenous AA (not shown). Another possibility was that the prevention resulted from a reduction in hydroxyeicosatetraenoic acid synthesis via the LOX pathways because of the decreased availability of unesterified AA in the double stable cells. We detected the expression of 15-LOX in 293 cells and found that the LOX inhibitor nordihydroguaiaretic acid induced apoptosis, as reported previously (16); moreover, addition of 15-hydroxyeicosatetraenoic acid apparently did not affect cell survival in 293 cells (not shown). Taken together, these results support the hypothesis that a high level of unesterified AA triggers apoptosis and its depletion blocks apoptosis. To obtain direct evidence, we next estimated the relative amounts of unesterified AA in the different cell lines by measuring the amount released into the medium (27). After induction of the overexpression, AA was reduced to 67, 61, and 26% in the FACL4, COX-2, and double stable cells, respectively, compared with their counterparts without induction (Fig. 4E).
Depletion of Arachidonic Acid Also Prevents TNF-α-Mediated Killing.
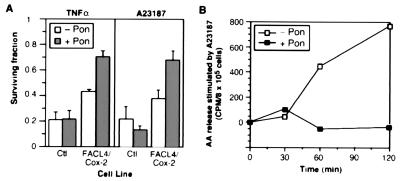
We next tested whether removal of AA could prevent cell death in other circumstances, for example, TNF-α-mediated killing. TNF-α exerts a cytotoxic effect against certain tumor cells, and release of AA by cPLA2 has been implicated in this process (13, 28). In the cells that overexpressed FACL4 or COX-2, the cell survival after TNF-α treatment was increased moderately (not shown), and it was significantly improved in the FACL4/COX-2 stable cells: the surviving fraction increased from 43.5 to 70.5% (n = 3, P < 0.001) (Fig. 5A). We observed that cell survival in the double stable cells was better than that in control cells even without induction. This probably resulted from a slight leak in promoter regulation of the cDNA constructs. We also examined cell survival in response to another agonist, Ca2+ ionophore A23187, which stimulates AA release by cPLA2 (29): survival was higher in induced FACL4 or COX-2 stable cells than in their uninduced counterparts (not shown). The improvement was even more pronounced in the double stable cells—cell survival rose from 37.8 to 67.8% (n = 3, P < 0.01) (Fig. 5A). We repeatedly observed synergistic actions by overexpression of both FACL4 and COX-2. Also, the COX-2-specific inhibitor (6-methoxy-2-naphthylacetic acid) partially abolished the improvement in cell survival in the double stable cells (not shown). To further test whether the improvement of cell survival was a consequence of reduction of the level of unesterified AA, we performed a pulse–chase experiment and measured the AA released to the medium after A23187 treatment. Control cells released a large amount of AA in response to A23187, but its release was completely abolished by overexpression of FACL4 and COX-2 (Fig. 5B). This result demonstrated that diversion of AA into metabolic pathways resulted in a low cellular concentration even under conditions of a strong stimulus for AA release. Therefore, an increase in the level of AA is a key step in TNF-α- and A23187-mediated killing and that unesterified AA mediates cell death signaling initiated by a variety of stimuli.
Figure 5.
Enzymatic removal of unesterified arachidonic acid protects against TNF-α- and calcium ionophore-mediated cell killing. (A) Determination of cell survival. The cells were induced (+ Pon) or not (− Pon) for 24 h, TNF-α (1 ng/ml) or the calcium ionophore A23187 (5 μM) was added, and the cells were incubated for another 72 h. The surviving fraction was determined and is plotted relative to that of the same line without TNF-α or A23187 treatment. Ctl, control cells harboring an empty vector. FACL4/COX-2, the double stable cells. (B) Measurement of the release of unesterified AA. The FACL4/COX-2 cells were prelabeled with [3H]AA, induced, and stimulated with A23187 (5 μM), and [3H]AA release was measured at the indicated time intervals. The values are from one experiment that is representative of four independent determinations.
The AA-Induced Apoptosis Is Suppressed by Antioxidants and Bcl-2.
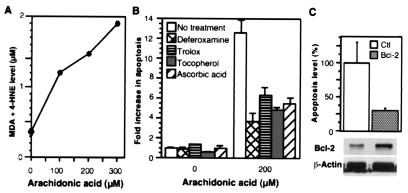
Lipid peroxidation often is a component of apoptosis (30), and we observed enhanced formation of malondialdehyde and 4-hydroxy-2(E)-nonenal in cells exposed to AA (Fig. 6A). Moreover, apoptosis decreased significantly when the cells were pretreated with antioxidants including deferoxamine, ascorbic acid, trolox, and tocopherol (Fig. 6B). We also tested apotransferrin, a membrane-impermeable antioxidant, and found that it did not alter AA-induced apoptosis (not shown). Thus, antioxidants partially protect against apoptosis by blocking an intracellular oxidative process. Our results suggest that reactive oxygen species are involved in AA-induced apoptosis and that they might play a role in peroxidation of the AA.
Figure 6.
Cells exposed to AA show increased lipid peroxidation; apoptosis is blocked by antioxidants and Bcl-2. (A) The level of lipid peroxidation products. The 293 cells were treated with AA for 48 h. The data presented are from one experiment that is representative of two independent experiments. MDA, malondialdehyde; 4-HNE, 4-hydroxynonenal (B) Attenuation of AA-induced apoptosis by antioxidants. Antioxidants were applied to 293 cells for 1 h, and then AA (200 μM) was added. Apoptosis was measured in triplicate determinations after 44 h and is shown relative to the level in control cells (in all treatments, P < 0.02). Concentrations of the antioxidants: deferoxamine, 60 μM; trolox, 100 μM; tocopherol, 100 μM; ascorbic acid, 200 μM. (C) Inhibition of AA-induced apoptosis by Bcl-2. Cells were transfected with an empty vector (Ctl) or with a cDNA encoding Bcl-2 (Bcl-2) and then were exposed to AA (300 μM). The values shown represent the means of triplicate determinations (P < 0.05). A portion of the transfectant was lysed, and the luciferase activity was determined to monitor the transfection efficiency. The expression level of Bcl-2 in the two lines was examined by immunoblotting (antibody from Boehringer Mannheim).
Bcl-2 is an antiapoptotic protein, and one of its effects is to suppress lipid peroxidation (30). We transfected cells with a Bcl-2 cDNA and found that it reduced the AA-induced apoptosis (Fig. 6C). This raised the possibility that the protection from apoptosis observed in the cells with an AA sink might have resulted from increased expression of Bcl-2. We found that the expression levels of Bcl-2 increased modestly when FACL4 and/or COX-2 were overexpressed (not shown). Thus, the apoptotic pathway initiated by AA involves lipid peroxidation and can be suppressed by Bcl-2, but the protective effect provided by expression of AA-metabolizing enzymes lies upstream of these steps—by regulating the amount of unesterified AA available.
Discussion
We have shown that unesterified AA is a critical signal for apoptosis and that the induction of apoptosis by NSAIDs and other inhibitors of AA metabolism is a consequence of its accumulation. Conversely, overexpression of COX-2 and FACL4, as is seen in colon and perhaps, other cancers, depletes unesterified AA, thereby removing a proapoptotic signal and promoting carcinogenesis. Our results suggest that pharmacological manipulation of the cellular level of unesterified AA to induce apoptosis could be a general approach to killing transformed cells.
The concentrations of AA that induced apoptosis in our in vitro experiments are higher than the steady-state levels in normal biological fluids, but the intracellular concentration is unknown and might approach such levels transiently under certain circumstances in vivo. For example, 5–20 nmol of AA are released from 109 platelets within 1 min upon stimulation by thrombin (31). Nonetheless, this response is more appropriately viewed as an in vitro model for testing the hypothesis, and, perhaps, mimicking a pathological response. The induction of apoptosis is not a detergent effect because other fatty acids, such as oleic and palmitic acids, did not cause the same response. Moreover, the AA-initiated apoptosis can be inhibited by removal of AA metabolically as shown in our experiments in which COX-2 and FACL4 were overexpressed.
COX-2-specific inhibitors SC-58125 and NS398 have been reported to enhance the induction of apoptosis in colon and prostate cancer cells by down-regulating Bcl-2 (32, 33), whereas 15-LOX was found to block apoptosis by up-regulating Bcl-2 (34). Along the same line, we here reported that overexpression of COX-2 and/or FACL4 blocked apoptosis and was paralleled by increased expression of Bcl-2. In addition, overexpression of Bcl-2 suppressed the AA-induced apoptosis. These results suggest that the expression of Bcl-2 is regulated by the level of unesterified AA and that the Bcl-2-dependent pathways play a protective role in AA-signaled apoptosis.
The cell-killing effect of TNF-α has been shown to signal through ceramide, and AA stimulated this pathway under different conditions (17, 28). To evaluate the involvement of ceramide, we measured the cellular ceramide level after exposure of the cells to exogenous AA (300 μM) for 1, 4, and 24 h. At 1 h there was a small increase (less than 2-fold) and at the other times there was no change compared with control cells (not shown). Thus, our results did not implicate ceramide as a site for the action of AA under these conditions.
It has been debated whether the augmented AA metabolism is an initiator or a consequence of tumor growth. The studies with COX-2 and its inhibitors strongly support the notion that the induction of COX-2 is a critical step in carcinogenesis and tumor progression. The proneoplastic effects of activating COX-2 and other AA metabolic pathways are several, but one important mechanism is the inhibition of apoptosis. In the case of colon physiology, apoptosis is a normal event to terminate the life cycle of intestinal epithelial cells. The diversion of AA by the induced enzymes in colon cancer lowers the level of unesterified AA and thereby promotes tumor growth by attenuating apoptosis. Specific eicosanoid products may also contribute to the transformation through enhancement of cell adhesion and proliferation. Nonetheless, the inhibition of apoptosis by coordinated activation of metabolic pathways that consume AA is a vital mechanism to promote tumor growth, and our findings indicate that development of specific and nontoxic inhibitors targeted to the AA-metabolizing pathways may provide novel approaches for therapeutic intervention.
Acknowledgments
We thank Dr. Stanley Korsmeyer for the Bcl-2 cDNA construct. We are grateful to Kavita Dave for technical assistance and Diana Lim for preparing the figures. This work was supported by a postdoctoral fellowship to Y.C. from the National Institutes of Health (HL 09775) and by grants to S.M.P. from the National Cancer Institute (CA 73992 and CA 42014).
Abbreviations
- AA
arachidonic acid
- COX-2
cyclooxygenase-2
- NSAIDs
nonsteroidal antiinflammatory drugs
- LOX
lipoxygenase
- cPLA2
cytosolic phospholipase A2
- TNF
tumor necrosis factor
- FACL
fatty acid-CoA ligase
- PG
prostaglandin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200367597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200367597
References
- 1.Tsujii M, Dubois R N. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 2.Sheng H, Shao J, Kirkland S C, Isakson P, Coffey R J, Morrow J, Beauchamp R D, DuBois R N. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott S M, White R L. Cell. 1996;87:783–786. doi: 10.1016/s0092-8674(00)81983-2. [DOI] [PubMed] [Google Scholar]
- 4.Thun M J, Namboodiri M M, Heath C W. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 5.Giardiello F M, Hamilton S R, Krush A J, Piantadosi S, Hylind L M, Celano P, Booker S V, Robinson C R, Offerhaus G J. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 6.Williams C S, Mann M, DuBois R N. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 7.Oshima M, Dinchuk J E, Kargman S L, Oshima H, Hancock B, Kwong E, Trzaskos J M, Evans J F, Taketo M M. Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 8.Kutchera W, Jones D A, Matsunami N, Groden J, McIntyre T M, Zimmerman G A, White R L, Prescott S M. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberhart C E, Coffey R J, Radhika A, Giardiello F M, Ferrenbach S, DuBois R N. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 10.Ikawa H, Kamitani H, Calvo B F, Foley J F, Eling T E. Cancer Res. 1999;59:360–366. [PubMed] [Google Scholar]
- 11.Hakumaki J M, Poptani H, Sandmair A M, Yla-Herttuala S, Kauppinen R A. Nat Med. 1999;5:1323–1327. doi: 10.1038/15279. [DOI] [PubMed] [Google Scholar]
- 12.Abramson S B, Leszczynska-Piziak J, Weissmann G. J Immunol. 1991;147:231–236. [PubMed] [Google Scholar]
- 13.Hayakawa M, Ishida N, Takeuchi K, Shibamoto S, Hori T, Oku N, Ito F, Tsujimoto M. J Biol Chem. 1993;268:11290–11295. [PubMed] [Google Scholar]
- 14.Sapirstein A, Spech R A, Witzgall R, Bonventre J V. J Biol Chem. 1996;271:21505–21513. doi: 10.1074/jbc.271.35.21505. [DOI] [PubMed] [Google Scholar]
- 15.Reid T, Ramesha C S, Ringold G M. J Biol Chem. 1991;266:16580–16586. [PubMed] [Google Scholar]
- 16.Tang D G, Chen Y Q, Honn K V. Proc Natl Acad Sci USA. 1996;93:5241–5246. doi: 10.1073/pnas.93.11.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan T A, Morin P J, Vogelstein B, Kinzler K W. Proc Natl Acad Sci USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surette M E, Winkler J D, Fonteh A N, Chilton F H. Biochemistry. 1996;35:9187–9196. doi: 10.1021/bi9530245. [DOI] [PubMed] [Google Scholar]
- 19.Surette M E, Fonteh A N, Bernatchez C, Chilton F H. Carcinogenesis. 1999;20:757–763. doi: 10.1093/carcin/20.5.757. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y, Traer E, Zimmerman G A, McIntyre T M, Prescott S M. Genomics. 1998;49:327–330. doi: 10.1006/geno.1998.5268. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y, Murphy K J, McIntyre T M, Zimmerman G A, Prescott S M. FEBS Lett. 2000;467:263–267. doi: 10.1016/s0014-5793(00)01159-5. [DOI] [PubMed] [Google Scholar]
- 22.Wilson D B, Prescott S M, Majerus P W. J Biol Chem. 1982;257:3510–3515. [PubMed] [Google Scholar]
- 23.Dole V, Meinertz H. J Biol Chem. 1960;235:2595–2599. [PubMed] [Google Scholar]
- 24.Smith W L, Garavito R M, DeWitt D L. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 25.Kang M J, Fujino T, Sasano H, Minekura H, Yabuki N, Nagura H, Iijima H, Yamamoto T T. Proc Natl Acad Sci USA. 1997;94:2880–2884. doi: 10.1073/pnas.94.7.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meade E A, Smith W L, DeWitt D L. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 27.Jayadev S, Linardic C M, Hannun Y A. J Biol Chem. 1994;269:5757–5763. [PubMed] [Google Scholar]
- 28.Jayadev S, Hayter H L, Andrieu N, Gamard C J, Liu B, Balu R, Hayakawa M, Ito F, Hannun Y A. J Biol Chem. 1997;272:17196–18203. doi: 10.1074/jbc.272.27.17196. [DOI] [PubMed] [Google Scholar]
- 29.Lin L-L, Lin A Y, Knopf J L. Proc Natl Acad Sci USA. 1992;89:6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 31.Neufeld E J, Majerus P W. J Biol Chem. 1983;258:2461–2467. [PubMed] [Google Scholar]
- 32.Sheng H, Shao J, Morrow J D, Beauchamp R D, DuBois R N. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 33.Liu X H, Yao S, Kirschenbaum A, Levine A C. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 34.Nishio E, Watanabe Y. Br J Pharmacol. 1997;122:1516–1522. doi: 10.1038/sj.bjp.0701529. [DOI] [PMC free article] [PubMed] [Google Scholar]