Abstract
Aims: To evaluate growth from diagnosis until final height (FH) in 21-hydroxylase deficiency patients.
Methods: A retrospective longitudinal study was performed. Only patients treated with hydrocortisone and fludrocortisone (in case of salt wasting) were evaluated. This resulted in a sample of 34 (21 male, 13 female) salt wasting patients (SW) and 26 (13 male, 13 female) non-salt wasting patients (NSW). Auxological data were compared to recent Dutch reference values.
Results: In the first three months of life, the mean length SDS decreased to -1.50, probably because of the high average glucocorticoid dose (40 mg/m2/day). FH corrected for target height (FHcorrTH) was -1.25 and -1.27 SDS in females and males, respectively. Patients treated with salt supplements during the first year, had a better FHcorrTH (-0.83 SDS). In NSW patients, FHcorrTH was -0.96 and -1.51 SDS in females and males, respectively. In SW and NSW, age at onset of puberty was within normal limits, but bone age was advanced. Mean pubertal height gain was reduced in males. Body mass index was only increased in NSW females.
Conclusion: In SW, loss of final height potential might be a result of glucocorticoid excess in the first three months and sodium depletion during infancy. In NSW, loss of FH potential was caused by the delay in diagnosis. In SW and NSW, the advanced bone age at onset of puberty (undertreatment in prebertal years) resulted in loss of height gain during puberty. The effect of intensive sodium chloride support in early infancy should be examined prospectively. Neonatal screening is required if the height prognosis in NSW patients is to be improved.
Full Text
The Full Text of this article is available as a PDF (194.1 KB).
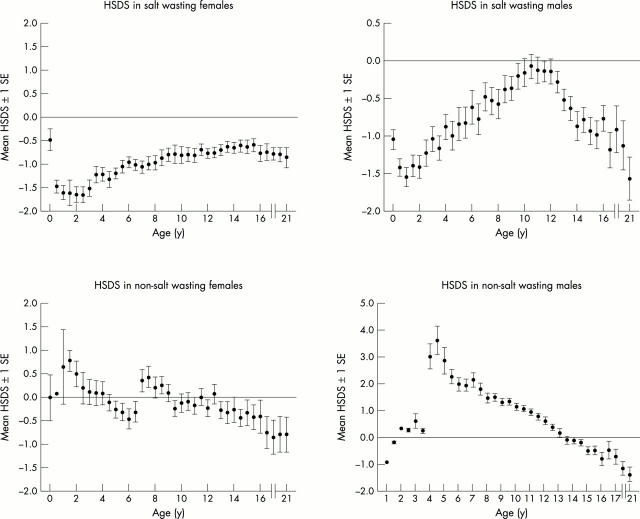
Figure 1 .
Longitudinal length and height SDS registration in patients (n = 60) with congenital adrenal hyperplasia according to reference values of Dutch children.9 Length SDS before the age of 2 years is recorded as height SDS. In non-salt wasting females there is an interruption in the mean HSDS at the age of 6.5 years, due to inclusion of four newly diagnosed late onset CAH females. Final height was expressed as SDS using the population average at 21 years of age. HSDS at 21 years was not corrected for target height.
Figure 2 .
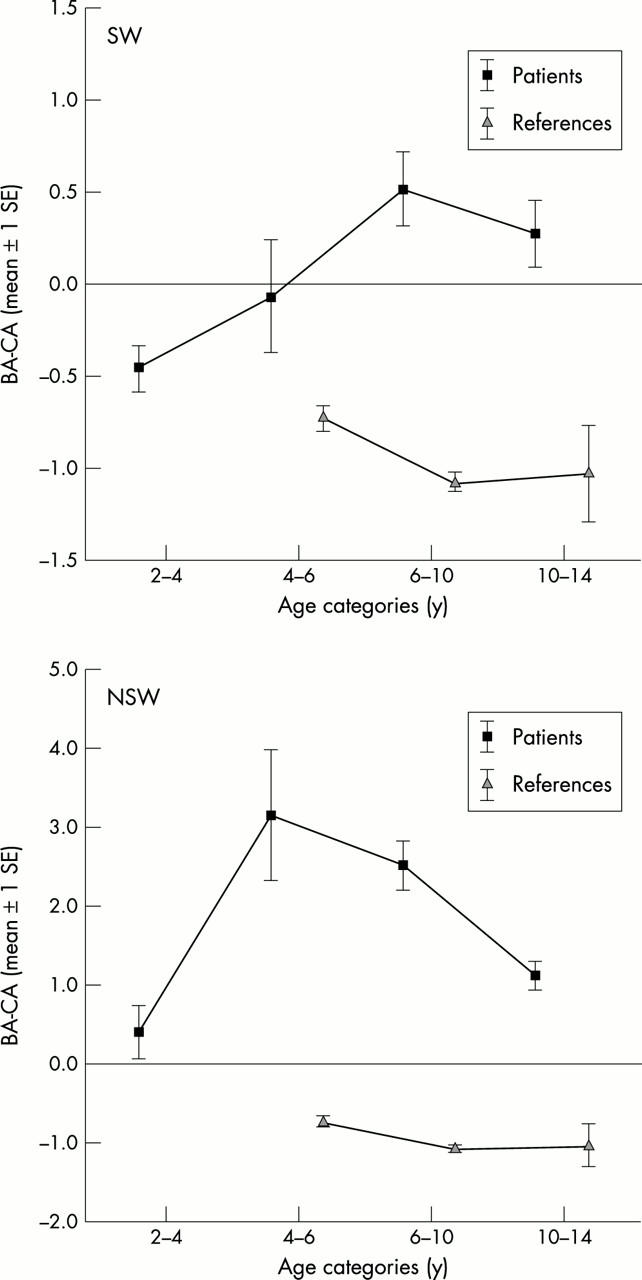
The difference between bone age (BA) according to G&P and chronological age (CA) in relation to age categories. The reference values were adjusted from Dutch TW2-RUS reference values.11 Bone age according to G&P is 0.8 years lower than that according to TW2-RUS (see results).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boepple P. A., Mansfield M. J., Link K., Crawford J. D., Crigler J. F., Jr, Kushner D. C., Blizzard R. M., Crowley W. F., Jr Impact of sex steroids and their suppression on skeletal growth and maturation. Am J Physiol. 1988 Oct;255(4 Pt 1):E559–E566. doi: 10.1152/ajpendo.1988.255.4.E559. [DOI] [PubMed] [Google Scholar]
- Bourguignon J. P. Variations in duration of pubertal growth: a mechanism compensating for differences in timing of puberty and minimizing their effects on final height. Belgian Study Group for Paediatric Endocrinology. Acta Paediatr Scand Suppl. 1988;347:16–24. [PubMed] [Google Scholar]
- Clayton G. W. Patterns of growth from birth to maturity in infants and children with congenital adrenal hyperplasia. Acta Endocrinol Suppl (Copenh) 1986;279:295–304. doi: 10.1530/acta.0.112s295. [DOI] [PubMed] [Google Scholar]
- Cornean R. E., Hindmarsh P. C., Brook C. G. Obesity in 21-hydroxylase deficient patients. Arch Dis Child. 1998 Mar;78(3):261–263. doi: 10.1136/adc.78.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban N. V., Loughlin T., Yergey A. L., Zawadzki J. K., Booth J. D., Winterer J. C., Loriaux D. L. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab. 1991 Jan;72(1):39–45. doi: 10.1210/jcem-72-1-39. [DOI] [PubMed] [Google Scholar]
- Fredriks A. M., van Buuren S., Burgmeijer R. J., Meulmeester J. F., Beuker R. J., Brugman E., Roede M. J., Verloove-Vanhorick S. P., Wit J. M. Continuing positive secular growth change in The Netherlands 1955-1997. Pediatr Res. 2000 Mar;47(3):316–323. doi: 10.1203/00006450-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Fredriks A. M., van Buuren S., Wit J. M., Verloove-Vanhorick S. P. Body index measurements in 1996-7 compared with 1980. Arch Dis Child. 2000 Feb;82(2):107–112. doi: 10.1136/adc.82.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T., Müller H. G., Köhler W., Prader A., Largo R., Molinari L. An analysis of the mid-growth and adolescent spurts of height based on acceleration. Ann Hum Biol. 1985 Mar-Apr;12(2):129–148. doi: 10.1080/03014468500007631. [DOI] [PubMed] [Google Scholar]
- Hargitai G., Hosszú E., Halász Z., Sólyom J. Serum osteocalcin and insulin-like growth factor I levels in children with congenital adrenal hyperplasia. Horm Res. 1999;52(3):131–139. doi: 10.1159/000023449. [DOI] [PubMed] [Google Scholar]
- Hauffa B. P., Winter A., Stolecke H. Treatment and disease effects on short-term growth and adult height in children and adolescents with 21-hydroxylase deficiency. Klin Padiatr. 1997 Mar-Apr;209(2):71–77. doi: 10.1055/s-2008-1043931. [DOI] [PubMed] [Google Scholar]
- Jansen M., Wit J. M., van den Brande J. L. Reinstitution of mineralocorticoid therapy in congenital adrenal hyperplasia. Effects on control and growth. Acta Paediatr Scand. 1981 Mar;70(2):229–233. doi: 10.1111/j.1651-2227.1981.tb05547.x. [DOI] [PubMed] [Google Scholar]
- Jäskeläinen J., Voutilainen R. Growth of patients with 21-hydroxylase deficiency: an analysis of the factors influencing adult height. Pediatr Res. 1997 Jan;41(1):30–33. doi: 10.1203/00006450-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Keenan B. S., Holcombe J. H., Wilson D. P., Kirkland R. T., Potts E., Clayton G. W. Plasma renin activity and the response to sodium depletion in salt-losing congenital adrenal hyperplasia. Pediatr Res. 1982 Feb;16(2):118–122. doi: 10.1203/00006450-198202000-00008. [DOI] [PubMed] [Google Scholar]
- Knorr D., Hinrichsen de Lienau S. G. Persistent obesity and short final height after corticoid overtreatment for congenital adrenal hyperplasia (CAH) in infancy. Acta Paediatr Jpn. 1988;30 (Suppl):89–92. [PubMed] [Google Scholar]
- Kuhnle U., Rösler A., Pareira J. A., Gunzcler P., Levine L. S., New M. I. The effects of long-term normalization of sodium balance on linear growth in disorders with aldosterone deficiency. Acta Endocrinol (Copenh) 1983 Apr;102(4):577–582. doi: 10.1530/acta.0.1020577. [DOI] [PubMed] [Google Scholar]
- Largo R. H., Gasser T., Prader A., Stuetzle W., Huber P. J. Analysis of the adolescent growth spurt using smoothing spline functions. Ann Hum Biol. 1978 Sep;5(5):421–434. doi: 10.1080/03014467800003071. [DOI] [PubMed] [Google Scholar]
- Largo R. H., Prader A. Somatische Pubertätsentwicklung bei Mädchen. Monatsschr Kinderheilkd. 1987 Aug;135(8):479–484. [PubMed] [Google Scholar]
- Linder B. L., Esteban N. V., Yergey A. L., Winterer J. C., Loriaux D. L., Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr. 1990 Dec;117(6):892–896. doi: 10.1016/s0022-3476(05)80128-3. [DOI] [PubMed] [Google Scholar]
- Mullis P. E., Hindmarsh P. C., Brook C. G. Sodium chloride supplement at diagnosis and during infancy in children with salt-losing 21-hydroxylase deficiency. Eur J Pediatr. 1990 Nov;150(1):22–25. doi: 10.1007/BF01959473. [DOI] [PubMed] [Google Scholar]
- New M. I., Gertner J. M., Speiser P. W., del Balzo P. Growth and final height in classical and nonclassical 21-hydroxylase deficiency. Acta Paediatr Jpn. 1988;30 (Suppl):79–88. [PubMed] [Google Scholar]
- New M. I., Lorenzen F., Lerner A. J., Kohn B., Oberfield S. E., Pollack M. S., Dupont B., Stoner E., Levy D. J., Pang S. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab. 1983 Aug;57(2):320–326. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- Pang S. Congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 1997 Dec;26(4):853–891. doi: 10.1016/s0889-8529(05)70285-1. [DOI] [PubMed] [Google Scholar]
- Rasat R., Espiner E. A., Abbott G. D. Growth patterns and outcomes in congenital adrenal hyperplasia; effect of chronic treatment regimens. N Z Med J. 1995 Aug 11;108(1005):311–314. [PubMed] [Google Scholar]
- Ray P. E., Holliday M. A. Growth rate in infants with impaired renal function. J Pediatr. 1988 Sep;113(3):594–600. doi: 10.1016/s0022-3476(88)80661-9. [DOI] [PubMed] [Google Scholar]
- Ray P. E., Schambelan M., Hintz R., Ruley E. J., Harrah J., Holliday M. A. Plasma renin activity as a marker for growth failure due to sodium deficiency in young rats. Pediatr Nephrol. 1992 Nov;6(6):523–526. doi: 10.1007/BF00866491. [DOI] [PubMed] [Google Scholar]
- Rösler A., Levine L. S., Schneider B., Novogroder M., New M. I. The interrelationship of sodium balance, plasma renin activity and ACTH in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1977 Sep;45(3):500–512. doi: 10.1210/jcem-45-3-500. [DOI] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976 Mar;51(3):170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H., Marubini E., Resele L. F. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol. 1976 Mar;3(2):109–126. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- Thilén A., Woods K. A., Perry L. A., Savage M. O., Wedell A., Ritzén E. M. Early growth is not increased in untreated moderately severe 21-hydroxylase deficiency. Acta Paediatr. 1995 Aug;84(8):894–898. doi: 10.1111/j.1651-2227.1995.tb13788.x. [DOI] [PubMed] [Google Scholar]
- Yu A. C., Grant D. B. Adult height in women with early-treated congenital adrenal hyperplasia (21-hydroxylase type): relation to body mass index in earlier childhood. Acta Paediatr. 1995 Aug;84(8):899–903. doi: 10.1111/j.1651-2227.1995.tb13789.x. [DOI] [PubMed] [Google Scholar]
- van der Kamp H. J., Slijper F. M., Brandenburg H., de Muinck Keizer-Schrama S. M., Drop S. L., Molenaar J. C. Evaluation of young women with congenital adrenal hyperplasia: a pilot study. Horm Res. 1992;37 (Suppl 3):45–49. doi: 10.1159/000182400. [DOI] [PubMed] [Google Scholar]
- von Schnakenburg K., Bidlingmaier F., Knorr D. 17-hydroxyprogesterone, androstenedione, and testosterone in normal children and in prepubertal patients with congenital adrenal hyperplasia. Eur J Pediatr. 1980 May;133(3):259–267. doi: 10.1007/BF00496086. [DOI] [PubMed] [Google Scholar]