Abstract
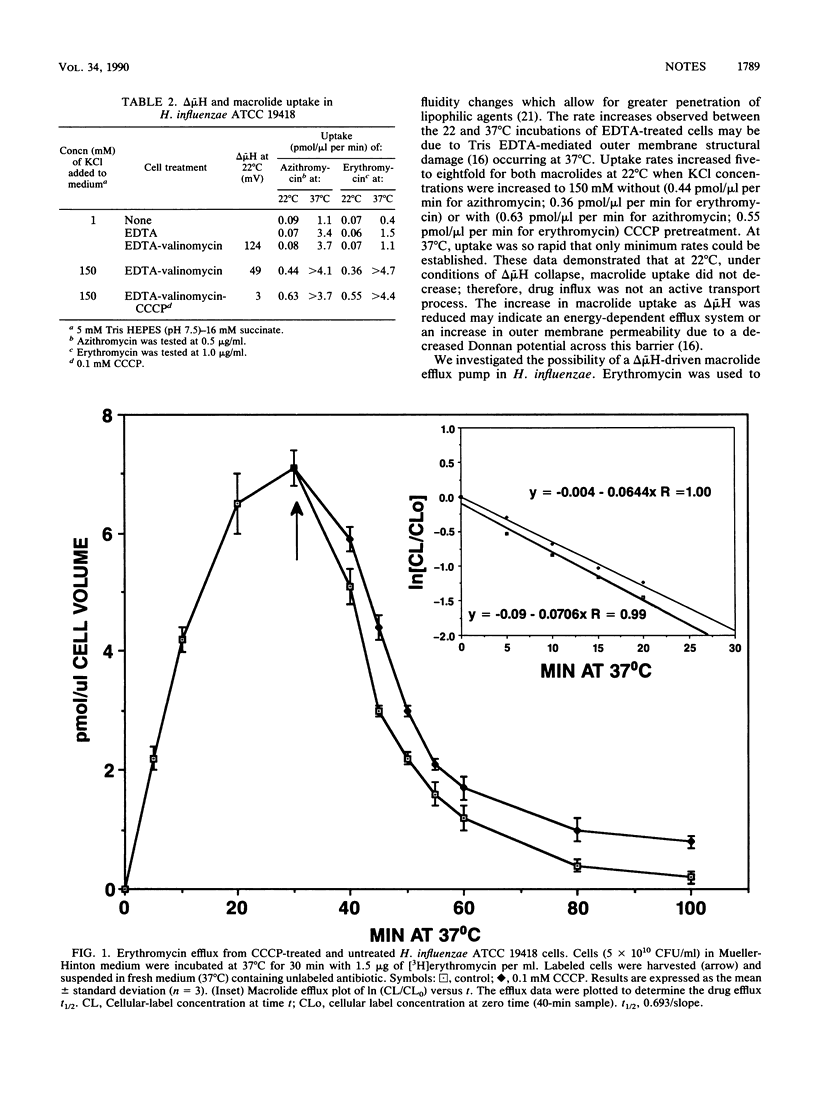
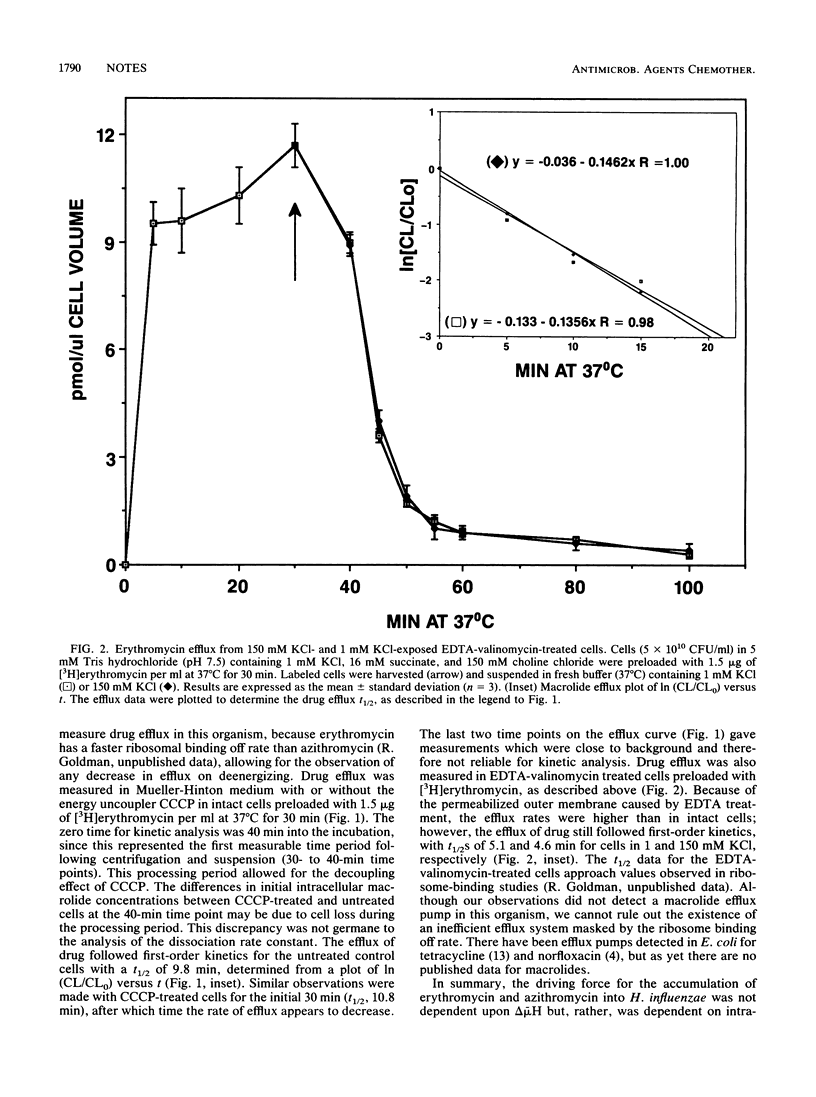
The effect of collapsing the electrochemical proton gradient (delta mu H) on [3H]erythromycin and [14C]azithromycin transport in Haemophilus influenzae ATCC 19418 was studied. The proton gradient and membrane potential were determined from the distribution of [2-14C]dimethadione and rubidium-86, respectively. delta mu H was reduced from 124 to 3 mV in EDTA-valinomycin-treated cells at 22 degrees C with 150 mM KCl and 0.1 mM carbonyl cyanide m-chlorophenylhydrazone. During the collapse of delta mu H, macrolide uptake increased. Erythromycin efflux studies strongly suggested that this increase was not due to an energy-dependent efflux pump but was likely due to increased outer membrane permeability. These data indicated that macrolide entry was not a delta mu H-driven active transport process but rather a passive diffusion process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P. Membrane potential in a potassium transport-negative mutant of Escherichia coli K-12. The distribution of rubidium in the presence of valinomycin indicates a higher potential than that of the tetraphenylphosphonium cation. Biochim Biophys Acta. 1982 Sep 15;681(3):474–483. doi: 10.1016/0005-2728(82)90190-6. [DOI] [PubMed] [Google Scholar]
- Barre J., Fournet M. P., Zini R., Deforges L., Duval J., Tillement J. P. In vitro [3H]-erythromycin binding to Staphylococcus aureus. Biochem Pharmacol. 1986 Mar 15;35(6):1001–1004. doi: 10.1016/0006-2952(86)90090-0. [DOI] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dette G. A., Knothe H., Kaula S. Modifying effects of pH and temperature on (14C)erythromycin uptake into Staphylococcus aureus--relation to antimicrobial activity. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jul;265(3-4):393–403. [PubMed] [Google Scholar]
- Fierro J. F., Hardisson C., Salas J. A. Involvement of cell impermeability in resistance to macrolides in some producer streptomycetes. J Antibiot (Tokyo) 1988 Jan;41(1):142–144. doi: 10.7164/antibiotics.41.142. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Pavlasová E., Baarda J. R. A transmembrane pH gradient in Streptococcus faecalis: origin, and dissipation by proton conductors and N,N'-dicyclohexylcarbodimide. Biochim Biophys Acta. 1970;196(2):235–244. doi: 10.1016/0005-2736(70)90011-8. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Lampson B. C., von David W., Parisi J. T. Novel mechanism for plasmid-mediated erythromycin resistance by pNE24 from Staphylococcus epidermidis. Antimicrob Agents Chemother. 1986 Nov;30(5):653–658. doi: 10.1128/aac.30.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. C., Putterman M. Accumulation in gram-postive and gram-negative bacteria as a mechanism of resistance to erythromycin. J Bacteriol. 1968 Mar;95(3):1111–1117. doi: 10.1128/jb.95.3.1111-1117.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Aronson D. A., Levy S. B. Susceptible Escherichia coli cells can actively excrete tetracyclines. Antimicrob Agents Chemother. 1983 Oct;24(4):544–551. doi: 10.1128/aac.24.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menninger J. R., Otto D. P. Erythromycin, carbomycin, and spiramycin inhibit protein synthesis by stimulating the dissociation of peptidyl-tRNA from ribosomes. Antimicrob Agents Chemother. 1982 May;21(5):811–818. doi: 10.1128/aac.21.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto Y., Bandoh K., Watanabe K., Katoh N., Ueno K. Macrolide accumulation by Bacteroides fragilis ATCC 25285. Antimicrob Agents Chemother. 1989 Feb;33(2):242–244. doi: 10.1128/aac.33.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Lorian V., Gerstein D., Loder P. B., Finland M. Enhancing effect on alkalinization of the medium on the activity of erythromycin against gram-negative bacteria. Appl Microbiol. 1968 Sep;16(9):1288–1292. doi: 10.1128/am.16.9.1288-1292.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman S. B., Jones N. R., Young F. E., Corcoran J. W. Sensitivity and resistance to erythromycin in Bacillus subtilis 168: the ribosomal binding of erythromycin and chloramphenicol. Biochim Biophys Acta. 1966 Aug 17;123(2):438–440. doi: 10.1016/0005-2787(66)90301-7. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Release of membrane components from viable Haemophilus parainfluenzae by ethylenediaminetetraacetic acid-tris(hydroxymethyl)-aminomethane. J Bacteriol. 1970 May;102(2):498–507. doi: 10.1128/jb.102.2.498-507.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]


