Abstract
Aim: To examine over time, the cellular response within the lungs of infants ventilated with respiratory syncytial virus (RSV) bronchiolitis and to compare this response in infants born at term with those born preterm.
Methods: Non-bronchoscopic bronchoalveolar lavage (BAL) samples were taken from 47 infants (24 born at term and 23 born preterm) who were ventilated for RSV positive bronchiolitis and 10 control infants. BAL cellularity and differential cell counts were calculated using standard techniques.
Results: Total cellularity in BAL over the first four days of ventilation in infants with RSV bronchiolitis was greater in term infants (median 2.2 (IQR 4.27) x 106 cells/ml) compared with preterm infants (0.58 (1.28) x 106 cells/ml). The magnitude of the cellular response in preterm infants with bronchiolitis was similar to that in the control group measured on day 1 (0.62 (0.77) x 106 cells/ml). BAL cellularity decreased progressively from the time of intubation in term infants, but remained relatively constant in preterm infants up to seven days after intubation.
Conclusions: There are differences in the magnitude and type of pulmonary cellular response in term and preterm infants ventilated with RSV bronchiolitis. The cellular response in term infants with bronchiolitis differs from that in a control group of infants. These differences may reflect variations in cellular recruitment in the lung and/or variations in airway calibre.
Full Text
The Full Text of this article is available as a PDF (186.2 KB).
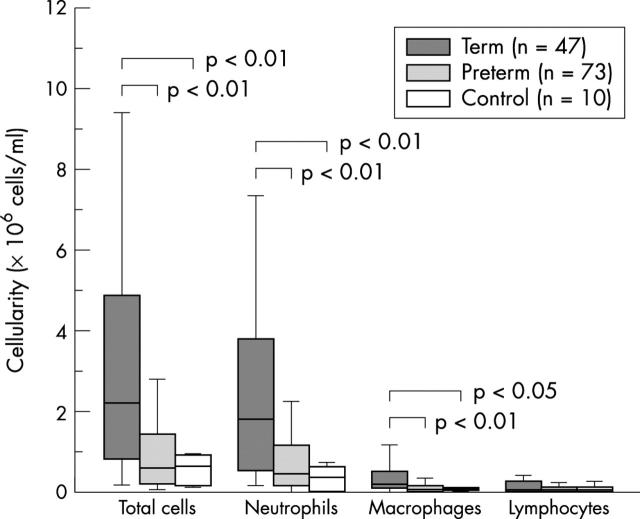
Figure 1 .
Total and individual leucocyte concentrations in term and preterm infants with bronchiolitis and the control group over days 1–4. Eosinophils were only seen in six samples, where they constituted less than 1% of the total. Necrotic and unidentifiable cells were not counted. All respiratory epithelial cells seen were non-viable.
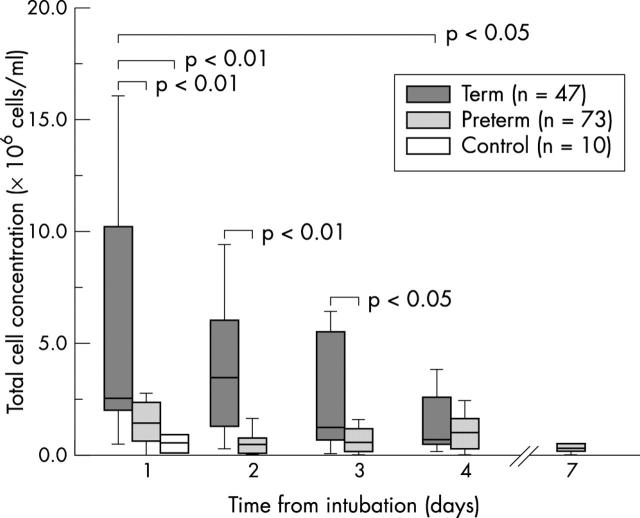
Figure 2 .
Total cell concentration over the first four days after intubation in term and preterm infants with RSV bronchiolitis. Data are also shown for control group infants (day 1) and six preterm infants still ventilated on day 7.
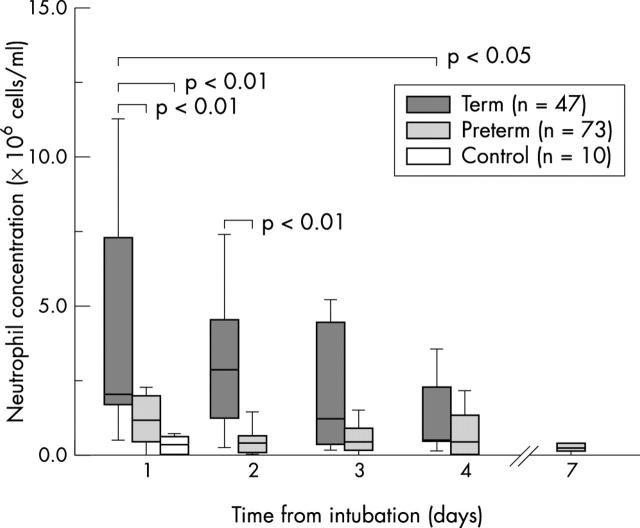
Figure 3 .
Neutrophil concentration over the first four days after intubation in term and preterm infants with RSV bronchiolitis. Data are also shown for control group infants (day 1) and six preterm infants still ventilated on day 7.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aherne W., Bird T., Court S. D., Gardner P. S., McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970 Feb;23(1):7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham Steven C., Jafri Hasan S., Bush Andrew J., Carubelli Cecilia M., Sheeran Paul, Hardy R. Doug, Ottolini Martin G., Ramilo Octavio, DeVincenzo John P. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. J Infect Dis. 2002 Apr 16;185(9):1222–1228. doi: 10.1086/340024. [DOI] [PubMed] [Google Scholar]
- Cherukupalli K., Larson J. E., Rotschild A., Thurlbeck W. M. Biochemical, clinical, and morphologic studies on lungs of infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1996 Oct;22(4):215–229. doi: 10.1002/(SICI)1099-0496(199610)22:4<215::AID-PPUL1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Eriksson M., Bennet R., Rotzén-Ostlund M., von Sydow M., Wirgart B. Zweygberg. Population-based rates of severe respiratory syncytial virus infection in children with and without risk factors, and outcome in a tertiary care setting. Acta Paediatr. 2002;91(5):593–598. doi: 10.1080/080352502753711740. [DOI] [PubMed] [Google Scholar]
- Everard M. L., Swarbrick A., Wrightham M., McIntyre J., Dunkley C., James P. D., Sewell H. F., Milner A. D. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994 Nov;71(5):428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin R., Anderson B., Percival T. Management of severe bronchiolitis: indications for ventilator support. N Z Med J. 1996 Apr 26;109(1020):137–139. [PubMed] [Google Scholar]
- Greensill Julie, McNamara Paul S., Dove Winifred, Flanagan Brian, Smyth Rosalind L., Hart C. Anthony. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003 Mar;9(3):372–375. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T., Pennycook A., Openshaw P. J. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001 Sep;31(9):2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Jobe A. J. The new BPD: an arrest of lung development. Pediatr Res. 1999 Dec;46(6):641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kimpen J. L., Ogra P. L. T cell redistribution kinetics after secondary infection of BALB/c mice with respiratory syncytial virus. Clin Exp Immunol. 1993 Jan;91(1):78–82. doi: 10.1111/j.1365-2249.1993.tb03358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. J., Gardner P. S., McQuillin J. Epidemiology of respiratory viral infection among paediatric inpatients over a six-year period in north-east England. Lancet. 1978 Nov 11;2(8098):1035–1038. doi: 10.1016/s0140-6736(78)92351-6. [DOI] [PubMed] [Google Scholar]
- Navas L., Wang E., de Carvalho V., Robinson J. Improved outcome of respiratory syncytial virus infection in a high-risk hospitalized population of Canadian children. Pediatric Investigators Collaborative Network on Infections in Canada. J Pediatr. 1992 Sep;121(3):348–354. doi: 10.1016/s0022-3476(05)90000-0. [DOI] [PubMed] [Google Scholar]
- Nievas F. Fernandez, Chernick V. Bronchopulmonary dysplasia (chronic lung disease of infancy): an update for the pediatrician. Clin Pediatr (Phila) 2002 Mar;41(2):77–85. doi: 10.1177/000992280204100202. [DOI] [PubMed] [Google Scholar]
- Openshaw P. J. Flow cytometric analysis of pulmonary lymphocytes from mice infected with respiratory syncytial virus. Clin Exp Immunol. 1989 Feb;75(2):324–328. [PMC free article] [PubMed] [Google Scholar]
- Openshaw Peter J. M. Potential therapeutic implications of new insights into respiratory syncytial virus disease. Respir Res. 2002;3 (Suppl 1):S15–S20. doi: 10.1186/rr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shann F., Pearson G., Slater A., Wilkinson K. Paediatric index of mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med. 1997 Feb;23(2):201–207. doi: 10.1007/s001340050317. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Wang S. Z., Dowling K. D., Forsyth K. D. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatr Child Health. 2001 Apr;37(2):146–151. doi: 10.1046/j.1440-1754.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hayle A. J. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985 Dec;66(Pt 12):2533–2538. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- Taylor G., Thomas L. H., Stott E. J. Effect of vaccination on cell populations in lung washes from calves after infection with respiratory syncytial virus. Res Vet Sci. 1989 Sep;47(2):231–235. [PubMed] [Google Scholar]
- Wang S. Z., Forsyth K. D. The interaction of neutrophils with respiratory epithelial cells in viral infection. Respirology. 2000 Mar;5(1):1–10. doi: 10.1046/j.1440-1843.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Wang S. Z., Hallsworth P. G., Dowling K. D., Alpers J. H., Bowden J. J., Forsyth K. D. Adhesion molecule expression on epithelial cells infected with respiratory syncytial virus. Eur Respir J. 2000 Feb;15(2):358–366. doi: 10.1034/j.1399-3003.2000.15b23.x. [DOI] [PubMed] [Google Scholar]
- Wang S. Z., Xu H., Wraith A., Bowden J. J., Alpers J. H., Forsyth K. D. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur Respir J. 1998 Sep;12(3):612–618. doi: 10.1183/09031936.98.12030612. [DOI] [PubMed] [Google Scholar]
- de Blic J., Midulla F., Barbato A., Clement A., Dab I., Eber E., Green C., Grigg J., Kotecha S., Kurland G. Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. European Respiratory Society. Eur Respir J. 2000 Jan;15(1):217–231. doi: 10.1183/09031936.00.15121700. [DOI] [PubMed] [Google Scholar]
- de Sierra T. M., Kumar M. L., Wasser T. E., Murphy B. R., Subbarao E. K. Respiratory syncytial virus-specific immunoglobulins in preterm infants. J Pediatr. 1993 May;122(5 Pt 1):787–791. doi: 10.1016/s0022-3476(06)80027-2. [DOI] [PubMed] [Google Scholar]
- van den Hoogen B. G., de Jong J. C., Groen J., Kuiken T., de Groot R., Fouchier R. A., Osterhaus A. D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001 Jun;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]