Abstract
Aims: To measure pimecrolimus blood concentrations and to evaluate tolerability and efficacy in children and infants treated topically for atopic dermatitis with pimecrolimus cream 1% for three weeks.
Methods: Three open label, non-controlled, multiple topical dose studies were conducted in children aged 8–14 years (study A, ten patients), and in infants aged 8–30 months (study B, eight patients) and 4–11 months (study C, eight patients). Pimecrolimus blood concentrations were determined on days 4 and 22 of treatment, and at end of study. Efficacy was assessed using the Eczema Area and Severity Index (EASI).
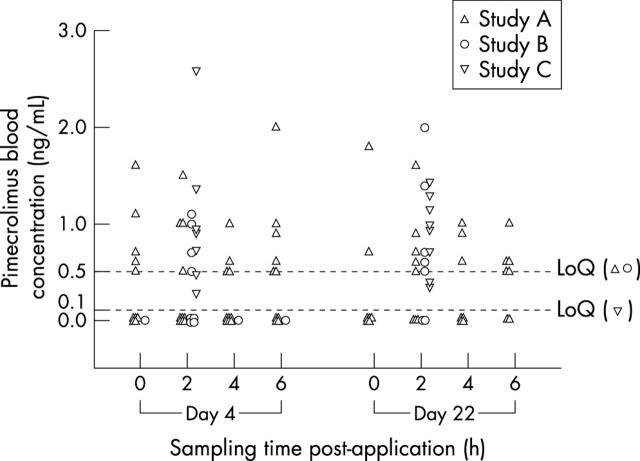
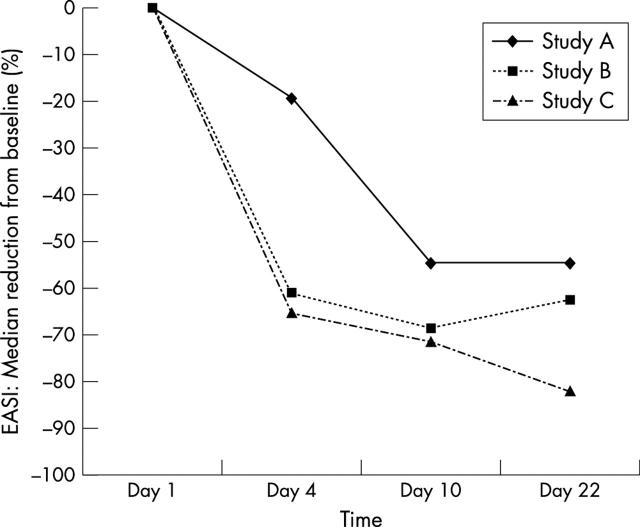
Results: Pimecrolimus blood concentrations were consistently low, typically (81%) below 1 ng/ml, with more than half of the measurements below the assay limit of quantitation (0.5 ng/ml) in studies A and B. The highest blood concentration measured throughout the three studies was 2.6 ng/ml. The cream was well tolerated, locally and systemically. The most common adverse event suspected to be related to study medication was a transient mild to moderate stinging sensation at the application site in 5/26 patients. There was no indication of any systemic adverse effect. The patients responded well to therapy with a rapid onset of action, usually within four days. Median reductions of EASI from baseline at day 22 were 55% (study A), 63% (study B), and 83% (study C).
Conclusion: Three weeks treatment of children and infants with extensive atopic dermatitis, using pimecrolimus cream 1% twice daily, is well tolerated and results in minimal systemic exposure, at which no systemic effect is expected.
Full Text
The Full Text of this article is available as a PDF (273.6 KB).
Figure 1 .
Pimecrolimus blood concentrations in children and infants on day 4 and day 22 of a three week treatment course.
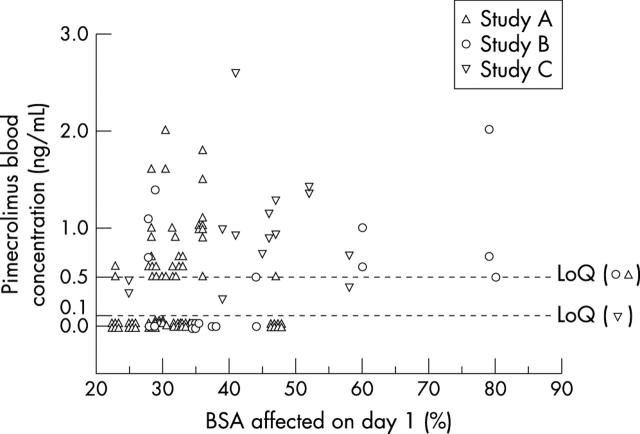
Figure 2 .
Blood concentrations of pimecrolimus versus body surface area at baseline (day 1).
Figure 3 .
Median reduction (%) of EASI from baseline (day 1) during three weeks of treatment of paediatric atopic dermatitis patients with pimecrolimus 1% cream.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eichenfield Lawrence F., Lucky Anne W., Boguniewicz Mark, Langley Richard G. B., Cherill Robert, Marshall Katharine, Bush Christopher, Graeber Michael. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002 Apr;46(4):495–504. doi: 10.1067/mjd.2002.122187. [DOI] [PubMed] [Google Scholar]
- Grassberger M., Baumruker T., Enz A., Hiestand P., Hultsch T., Kalthoff F., Schuler W., Schulz M., Werner F. J., Winiski A. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999 Aug;141(2):264–273. doi: 10.1046/j.1365-2133.1999.02974.x. [DOI] [PubMed] [Google Scholar]
- Hanifin J. M., Thurston M., Omoto M., Cherill R., Tofte S. J., Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001 Feb;10(1):11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- Harper J., Green A., Scott G., Gruendl E., Dorobek B., Cardno M., Burtin P. First experience of topical SDZ ASM 981 in children with atopic dermatitis. Br J Dermatol. 2001 Apr;144(4):781–787. doi: 10.1046/j.1365-2133.2001.04133.x. [DOI] [PubMed] [Google Scholar]
- Hebert A. A., Warken K. A., Cherill R. Pimecrolimus cream 1%: a new development in nonsteroid topical treatment of inflammatory skin diseases. Semin Cutan Med Surg. 2001 Dec;20(4):260–267. doi: 10.1053/sder.2001.29062. [DOI] [PubMed] [Google Scholar]
- Ho Vincent C., Gupta Aditya, Kaufmann Roland, Todd Gail, Vanaclocha Francisco, Takaoka Roberto, Fölster-Holst Regina, Potter Paul, Marshall Katherine, Thurston Mark. Safety and efficacy of nonsteroid pimecrolimus cream 1% in the treatment of atopic dermatitis in infants. J Pediatr. 2003 Feb;142(2):155–162. doi: 10.1067/mpd.2003.65. [DOI] [PubMed] [Google Scholar]
- Kapp Alexander, Papp Kim, Bingham Ann, Fölster-Holst Regina, Ortonne Jean-Paul, Potter Paul C., Gulliver Wayne, Paul Carle, Molloy Stephen, Barbier Nathalie. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug. J Allergy Clin Immunol. 2002 Aug;110(2):277–284. doi: 10.1067/mai.2002.126500. [DOI] [PubMed] [Google Scholar]
- Luger T., Van Leent E. J., Graeber M., Hedgecock S., Thurston M., Kandra A., Berth-Jones J., Bjerke J., Christophers E., Knop J. SDZ ASM 981: an emerging safe and effective treatment for atopic dermatitis. Br J Dermatol. 2001 Apr;144(4):788–794. doi: 10.1046/j.1365-2133.2001.04134.x. [DOI] [PubMed] [Google Scholar]
- Meingassner J. G., Grassberger M., Fahrngruber H., Moore H. D., Schuurman H., Stütz A. A novel anti-inflammatory drug, SDZ ASM 981, for the topical and oral treatment of skin diseases: in vivo pharmacology. Br J Dermatol. 1997 Oct;137(4):568–576. doi: 10.1111/j.1365-2133.1997.tb03788.x. [DOI] [PubMed] [Google Scholar]
- Paul C., Graeber M., Stuetz A. Ascomycins: promising agents for the treatment of inflammatory skin diseases. Expert Opin Investig Drugs. 2000 Jan;9(1):69–77. doi: 10.1517/13543784.9.1.69. [DOI] [PubMed] [Google Scholar]
- Queille-Roussel C., Graeber M., Thurston M., Lachapelle J. M., Decroix J., de Cuyper C., Ortonne J. P. SDZ ASM 981 is the first non-steroid that suppresses established nickel contact dermatitis elicited by allergen challenge. Contact Dermatitis. 2000 Jun;42(6):349–350. doi: 10.1034/j.1600-0536.2000.042006349.x. [DOI] [PubMed] [Google Scholar]
- Queille-Roussel C., Paul C., Duteil L., Lefebvre M. C., Rapatz G., Zagula M., Ortonne J. P. The new topical ascomycin derivative SDZ ASM 981 does not induce skin atrophy when applied to normal skin for 4 weeks: a randomized, double-blind controlled study. Br J Dermatol. 2001 Mar;144(3):507–513. doi: 10.1046/j.1365-2133.2001.04076.x. [DOI] [PubMed] [Google Scholar]
- Rajka G., Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–14. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- Rappersberger Klemens, Komar Michael, Ebelin Marie-Eve, Scott Graham, Burtin Pascale, Greig Gerard, Kehren Jeanne, Chibout Salah-Dine, Cordier Andre, Holter Wolfgang. Pimecrolimus identifies a common genomic anti-inflammatory profile, is clinically highly effective in psoriasis and is well tolerated. J Invest Dermatol. 2002 Oct;119(4):876–887. doi: 10.1046/j.1523-1747.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- Sampson H. A. Pathogenesis of eczema. Clin Exp Allergy. 1990 Sep;20(5):459–467. doi: 10.1111/j.1365-2222.1990.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Thaçi Diamant, Steinmeyer Katrin, Ebelin Marie-Eve, Scott Graham, Kaufmann Roland. Occlusive treatment of chronic hand dermatitis with pimecrolimus cream 1% results in low systemic exposure, is well tolerated, safe, and effective. An open study. Dermatology. 2003;207(1):37–42. doi: 10.1159/000070939. [DOI] [PubMed] [Google Scholar]
- Van Leent E. J., Gräber M., Thurston M., Wagenaar A., Spuls P. I., Bos J. D. Effectiveness of the ascomycin macrolactam SDZ ASM 981 in the topical treatment of atopic dermatitis. Arch Dermatol. 1998 Jul;134(7):805–809. doi: 10.1001/archderm.134.7.805. [DOI] [PubMed] [Google Scholar]
- Van Leent Edwin J. M., Ebelin Marie Eve, Burtin Pascale, Dorobek Birgit, Spuls Phyllis I., Bos Jan D. Low systemic exposure after repeated topical application of Pimecrolimus (Elidel), SD Z ASM 981) in patients with atopic dermatitis. Dermatology. 2002;204(1):63–68. doi: 10.1159/000051813. [DOI] [PubMed] [Google Scholar]
- Wellington K., Spencer C. M. Sdz asm 981. BioDrugs. 2000 Dec;14(6):409–416. doi: 10.2165/00063030-200014060-00005. [DOI] [PubMed] [Google Scholar]
- Williams H., Robertson C., Stewart A., Aït-Khaled N., Anabwani G., Anderson R., Asher I., Beasley R., Björkstén B., Burr M. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999 Jan;103(1 Pt 1):125–138. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]