Full Text
The Full Text of this article is available as a PDF (219.0 KB).
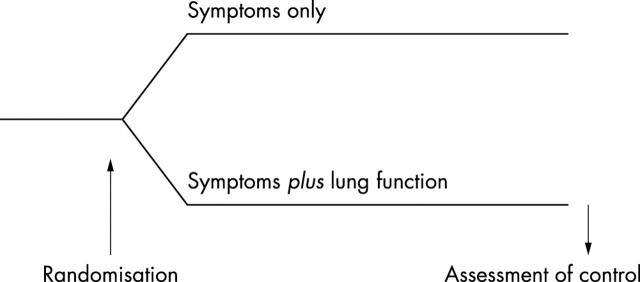
Figure 1 .
Design of the clinical trial needed to test the hypothesis that monitoring of lung function in childhood asthma is useful. Such studies are rare in children, and no such studies have been published showing usefulness of routine lung function monitoring.
Figure 2 .
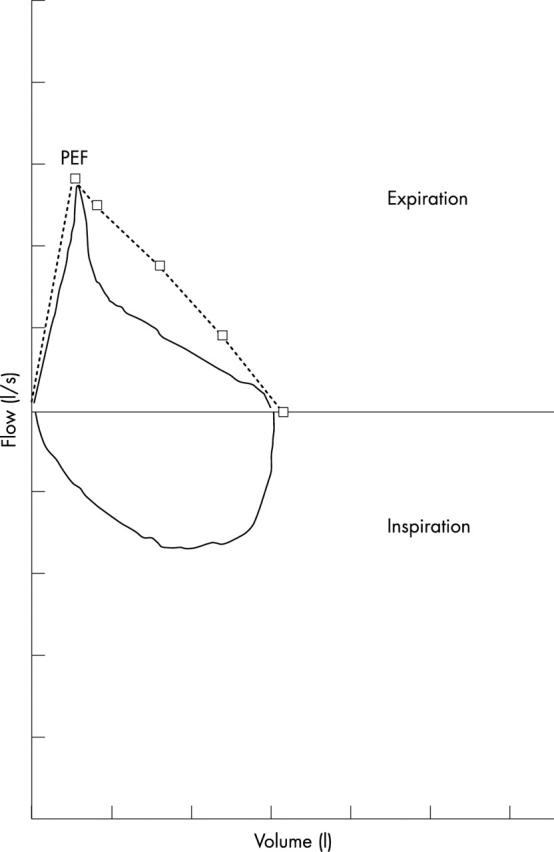
Flow-volume curve of a child with asthma, showing the characteristic concave expiratory pattern with markedly reduced mid-expiratory flow rates. The reference values for peak expiratory flow (PEF), mid-expiratory flows at 25%, 50%, and 75% (MEF25–75) of forced vital capacity (FVC), and the FVC itself are represented by squares, and are connected by a dashed line representing a hypothetical "normal" expiratory flow-volume curve. The FEV1 can not be read directly from a flow-volume curve because there is no time axis, but the spirometer software will provide it. In this case, the FEV1 was 71% of the predicted value. Note that despite considerable airways obstruction, PEF is normal.
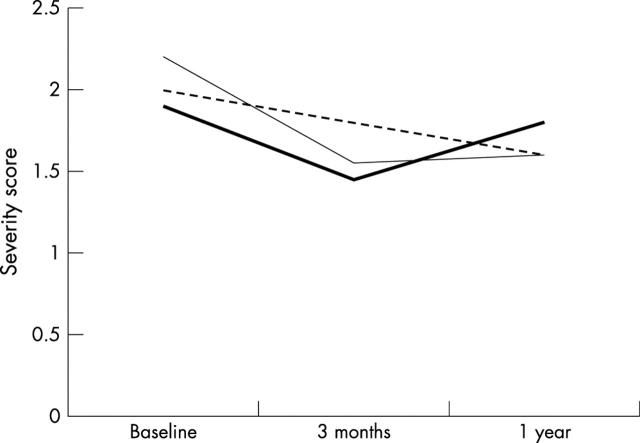
Figure 3 .
Asthma severity scores in three groups of asthmatic children, one monitoring symptoms only (dashed line), one monitoring peak expiratory flow (PEF) at home on a daily basis (thin solid line), and one monitoring PEF at home only when symptomatic for one year. Although there was a trend towards a difference in asthma severity between symptom only monitoring and PEF monitoring after three months (p = 0.07) this difference disappeared during further follow up (after Yoos et al24).
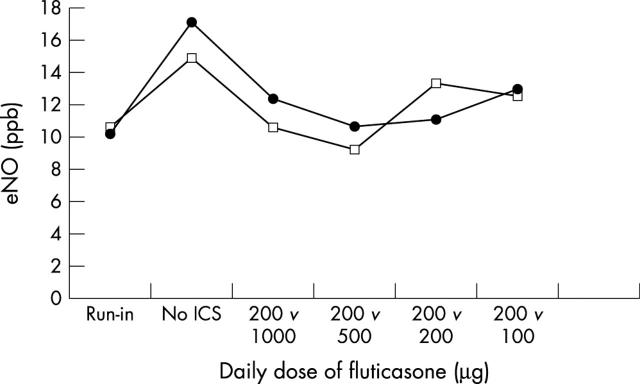
Figure 4 .
Nitric oxide levels in exhaled air (eNO) in asthmatic children, treated for one year with a constant dose of fluticasone (thin line, open squares) or a stepdown schedule with a high starting dose tapering off to a low maintenance dose (thick line, solid circles). Run-in: six week period during which all patients inhaled 200 µg/day fluticasone by dry powder inhaler. No ICS: wash-out period of 2–4 weeks during which no inhaled corticosteroids were used. The results show no effect of the dose of fluticasone on eNO levels. After Visser et al.50
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balfour-Lynn I. Difficult asthma: beyond the guidelines. Arch Dis Child. 1999 Feb;80(2):201–206. doi: 10.1136/adc.80.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola G., Amalfitano G., Tonolli E., Perazzoli C., Piacentini I., Mastella G. Burkholderia (Pseudomonas) cepacia epidemiology in a cystic fibrosis population: a genome finger-printing study. Acta Paediatr. 1996 May;85(5):554–557. doi: 10.1111/j.1651-2227.1996.tb14085.x. [DOI] [PubMed] [Google Scholar]
- Clough J. B., Holgate S. T. Episodes of respiratory morbidity in children with cough and wheeze. Am J Respir Crit Care Med. 1994 Jul;150(1):48–53. doi: 10.1164/ajrccm.150.1.8025771. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Cutz E., Levison H. Occult pulmonary abnormalities in asymptomatic asthmatic children. Chest. 1977 Mar;71(3):361–365. doi: 10.1378/chest.71.3.361. [DOI] [PubMed] [Google Scholar]
- Cowie R. L., Revitt S. G., Underwood M. F., Field S. K. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest. 1997 Dec;112(6):1534–1538. doi: 10.1378/chest.112.6.1534. [DOI] [PubMed] [Google Scholar]
- Crenesse D., Berlioz M., Bourrier T., Albertini M. Spirometry in children aged 3 to 5 years: reliability of forced expiratory maneuvers. Pediatr Pulmonol. 2001 Jul;32(1):56–61. doi: 10.1002/ppul.1089. [DOI] [PubMed] [Google Scholar]
- Eid N., Yandell B., Howell L., Eddy M., Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000 Feb;105(2):354–358. doi: 10.1542/peds.105.2.354. [DOI] [PubMed] [Google Scholar]
- Ferguson A. C., Whitelaw M., Brown H. Correlation of bronchial eosinophil and mast cell activation with bronchial hyperresponsiveness in children with asthma. J Allergy Clin Immunol. 1992 Oct;90(4 Pt 1):609–613. doi: 10.1016/0091-6749(92)90133-m. [DOI] [PubMed] [Google Scholar]
- Franklin P. J., Taplin R., Stick S. M. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999 Jan;159(1):69–73. doi: 10.1164/ajrccm.159.1.9804134. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge A. L., Kitch B. T., Paltiel A. D., Kuntz K. M., Neumann P. J., Dockery D. W., Weiss S. T. FEV(1) is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001 Jan;107(1):61–67. doi: 10.1067/mai.2001.111590. [DOI] [PubMed] [Google Scholar]
- Gannon P. F., Belcher J., Pantin C. F., Burge P. S. The effect of patient technique and training on the accuracy of self-recorded peak expiratory flow. Eur Respir J. 1999 Jul;14(1):28–31. doi: 10.1034/j.1399-3003.1999.14a07.x. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Springer C., Avital A., Godfrey S., Bar-Yishay E. Can peak expiratory flow measurements estimate small airway function in asthmatic children? Chest. 2001 Aug;120(2):482–488. doi: 10.1378/chest.120.2.482. [DOI] [PubMed] [Google Scholar]
- Greineder D. K., Loane K. C., Parks P. A randomized controlled trial of a pediatric asthma outreach program. J Allergy Clin Immunol. 1999 Mar;103(3 Pt 1):436–440. doi: 10.1016/s0091-6749(99)70468-9. [DOI] [PubMed] [Google Scholar]
- Grol M. H., Gerritsen J., Postma D. S. Asthma: from childhood to adulthood. Allergy. 1996 Dec;51(12):855–869. doi: 10.1111/j.1398-9995.1996.tb04485.x. [DOI] [PubMed] [Google Scholar]
- Jenkins M. A., Hopper J. L., Bowes G., Carlin J. B., Flander L. B., Giles G. G. Factors in childhood as predictors of asthma in adult life. BMJ. 1994 Jul 9;309(6947):90–93. doi: 10.1136/bmj.309.6947.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. L., Herbison P., Cowan J. O., Flannery E. M., Hancox R. J., McLachlan C. R., Taylor D. R. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose-response relationship. Eur Respir J. 2002 Sep;20(3):601–608. doi: 10.1183/09031936.02.00285302. [DOI] [PubMed] [Google Scholar]
- Jöbsis Q., Schellekens S. L., Kroesbergen A., Hop W. C., de Jongste J. C. Off-line sampling of exhaled air for nitric oxide measurement in children: methodological aspects. Eur Respir J. 2001 May;17(5):898–903. doi: 10.1183/09031936.01.17508980. [DOI] [PubMed] [Google Scholar]
- Kamps A. W., Brand P. L. Education, self-management and home peak flow monitoring in childhood asthma. Paediatr Respir Rev. 2001 Jun;2(2):165–169. doi: 10.1053/prrv.2000.0125. [DOI] [PubMed] [Google Scholar]
- Kamps A. W., Roorda R. J., Brand P. L. Peak flow diaries in childhood asthma are unreliable. Thorax. 2001 Mar;56(3):180–182. doi: 10.1136/thorax.56.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonov S. A., Barnes P. J. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001 Jun;163(7):1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- Madge P., McColl J., Paton J. Impact of a nurse-led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax. 1997 Mar;52(3):223–228. doi: 10.1136/thx.52.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male I., Richter H., Seddon P. Children's perception of breathlessness in acute asthma. Arch Dis Child. 2000 Oct;83(4):325–329. doi: 10.1136/adc.83.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. A., Bridge P. D., Pao C. S. Lung function tests for pre-school children. Paediatr Respir Rev. 2001 Mar;2(1):37–45. doi: 10.1053/prrv.2000.0100. [DOI] [PubMed] [Google Scholar]
- McKenzie S. A., Chan E., Dundas I., Bridge P. D., Pao C. S., Mylonopoulou M., Healy M. J. R. Airway resistance measured by the interrupter technique: normative data for 2-10 year olds of three ethnicities. Arch Dis Child. 2002 Sep;87(3):248–251. doi: 10.1136/adc.87.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkus P. J., Mijnsbergen J. Y., Hop W. C., de Jongste J. C. Interrupter resistance in preschool children: measurement characteristics and reference values. Am J Respir Crit Care Med. 2001 May;163(6):1350–1355. doi: 10.1164/ajrccm.163.6.2005076. [DOI] [PubMed] [Google Scholar]
- Pattemore P. K., Asher M. I., Harrison A. C., Mitchell E. A., Rea H. H., Stewart A. W. The interrelationship among bronchial hyperresponsiveness, the diagnosis of asthma, and asthma symptoms. Am Rev Respir Dis. 1990 Sep;142(3):549–554. doi: 10.1164/ajrccm/142.3.549. [DOI] [PubMed] [Google Scholar]
- Rasmussen Finn, Taylor D. Robin, Flannery Erin M., Cowan Jan O., Greene Justina M., Herbison G. Peter, Sears Malcolm R. Risk factors for hospital admission for asthma from childhood to young adulthood: a longitudinal population study. J Allergy Clin Immunol. 2002 Aug;110(2):220–227. doi: 10.1067/mai.2002.125295. [DOI] [PubMed] [Google Scholar]
- Rietveld S., Everaerd W. Perceptions of asthma by adolescents at home. Chest. 2000 Feb;117(2):434–439. doi: 10.1378/chest.117.2.434. [DOI] [PubMed] [Google Scholar]
- Ronchetti R., Indinnimeo L., Bonci E., Corrias A., Evans D., Hindi-Alexander M., Midulla F., Pulejo R., Villa M. P. Asthma self-management programmes in a population of Italian children: a multicentric study. Italian Study Group on Asthma Self-Management Programmes. Eur Respir J. 1997 Jun;10(6):1248–1253. doi: 10.1183/09031936.97.10061248. [DOI] [PubMed] [Google Scholar]
- Roorda R. J., Gerritsen J., van Aalderen W. M., Schouten J. P., Veltman J. C., Weiss S. T., Knol K. Follow-up of asthma from childhood to adulthood: influence of potential childhood risk factors on the outcome of pulmonary function and bronchial responsiveness in adulthood. J Allergy Clin Immunol. 1994 Mar;93(3):575–584. doi: 10.1016/s0091-6749(94)70069-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal Mark. Differential diagnosis of asthma. Paediatr Respir Rev. 2002 Jun;3(2):148–153. [PubMed] [Google Scholar]
- Sly P. D., Flack F. Is home monitoring of lung function worthwhile for children with asthma? Thorax. 2001 Mar;56(3):164–165. doi: 10.1136/thorax.56.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sont J. K., Willems L. N., Bel E. H., van Krieken J. H., Vandenbroucke J. P., Sterk P. J. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med. 1999 Apr;159(4 Pt 1):1043–1051. doi: 10.1164/ajrccm.159.4.9806052. [DOI] [PubMed] [Google Scholar]
- Taylor M. R. Asthma: audit of peak flow rate guidelines for admission and discharge. Arch Dis Child. 1994 May;70(5):432–434. doi: 10.1136/adc.70.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. O., Taylor D., Bennett R., Fitzgerald J. M. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med. 1998 Feb;157(2):540–546. doi: 10.1164/ajrccm.157.2.9703060. [DOI] [PubMed] [Google Scholar]
- Vilozni D., Barker M., Jellouschek H., Heimann G., Blau H. An interactive computer-animated system (SpiroGame) facilitates spirometry in preschool children. Am J Respir Crit Care Med. 2001 Dec 15;164(12):2200–2205. doi: 10.1164/ajrccm.164.12.2101002. [DOI] [PubMed] [Google Scholar]
- Visser M. J., Postma D. S., Arends L. R., de Vries T. W., Duiverman E. J., Brand P. L. One-year treatment with different dosing schedules of fluticasone propionate in childhood asthma. Effects on hyperresponsiveness, lung function, and height. Am J Respir Crit Care Med. 2001 Dec 1;164(11):2073–2077. doi: 10.1164/ajrccm.164.11.2103075. [DOI] [PubMed] [Google Scholar]
- Wensley D. C., Silverman M. The quality of home spirometry in school children with asthma. Thorax. 2001 Mar;56(3):183–185. doi: 10.1136/thorax.56.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseldine L. J., McCarthy P., Silverman M. Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Arch Dis Child. 1999 Feb;80(2):110–114. doi: 10.1136/adc.80.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoos H. Lorrie, Kitzman Harriet, McMullen Ann, Henderson Charles, Sidora Kimberly. Symptom monitoring in childhood asthma: a randomized clinical trial comparing peak expiratory flow rate with symptom monitoring. Ann Allergy Asthma Immunol. 2002 Mar;88(3):283–291. doi: 10.1016/S1081-1206(10)62010-8. [DOI] [PubMed] [Google Scholar]
- Zach M. S. The physiology of forced expiration. Paediatr Respir Rev. 2000 Mar;1(1):36–39. doi: 10.1053/prrv.2000.0010. [DOI] [PubMed] [Google Scholar]
- van Essen-Zandvliet E. E., Hughes M. D., Waalkens H. J., Duiverman E. J., Pocock S. J., Kerrebijn K. F. Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. The Dutch Chronic Non-specific Lung Disease Study Group. Am Rev Respir Dis. 1992 Sep;146(3):547–554. doi: 10.1164/ajrccm/146.3.547. [DOI] [PubMed] [Google Scholar]