Abstract
Background: A major consequence of malnutrition in cystic fibrosis (CF) patients is the loss of lean body mass (LBM) and the subsequent impairment of respiratory muscle function.
Aim: To determine whether insulin-like growth factor I (IGF-I) could be related to the LBM depletion and the evolution of respiratory disease in CF patients.
Methods: LBM was evaluated by dual energy x ray absorptiometry; serum concentrations of IGF-I were measured in 24 CF patients twice with a one year interval. Both values were expressed as SD score (SDS) calculated from normal data for age, sex, and pubertal stage and analysed with respect to anthropometric evaluation and disease related conditions.
Results: At the initial evaluation, IGF-I SDS had a mean value of -0.98 (range -3.6 to 3.2) and correlated with weight for age index, LBM SDS, and lung disease related conditions. Multiple regression analysis showed that only LBM remained independently related to IGF-I, suggesting that the relation of IGF-I to LBM was independent of weight and that the correlation between IGF-I and the respiratory conditions was related to the level of LBM. IGF-I SDS at the first evaluation was lower for the patients who lost ⩾5% of weight for age index or ⩾1 SD of LBM between the two evaluations.
Conclusion: Low levels of IGF-I could be crucial for clinical outcome by impairing LBM and respiratory function. IGF-I could be a tool for nutritional evaluation by identifying the CF patients at risk of LBM depletion.
Full Text
The Full Text of this article is available as a PDF (354.4 KB).
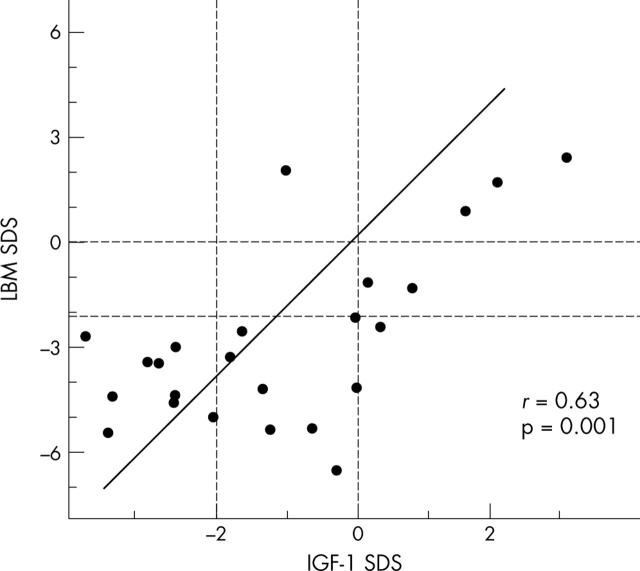
Figure 1 .
Relation between LBM SDS and IGF-I SDS observed at the initial evaluation.
Figure 2 .
Relation between the variation in weight at one year interval and IGF-I SDS at the initial evaluation.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Khenaizan S., Schechter J. F., Sasseville D. Pseudoporphyria induced by propionic acid derivatives. J Cutan Med Surg. 1999 Jan;3(3):162–166. doi: 10.1177/120347549900300314. [DOI] [PubMed] [Google Scholar]
- Arora N. S., Rochester D. F. Effect of body weight and muscularity on human diaphragm muscle mass, thickness, and area. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jan;52(1):64–70. doi: 10.1152/jappl.1982.52.1.64. [DOI] [PubMed] [Google Scholar]
- Arumugam R., LeBlanc A., Seilheimer D. K., Hardin D. S. Serum leptin and IGF-I levels in cystic fibrosis. Endocr Res. 1998 May;24(2):247–257. doi: 10.1080/07435809809135532. [DOI] [PubMed] [Google Scholar]
- Baur L. A., Waters D. L., Allen B. J., Blagojevic N., Gaskin K. J. Nitrogen deposition in malnourished children with cystic fibrosis. Am J Clin Nutr. 1991 Feb;53(2):503–511. doi: 10.1093/ajcn/53.2.503. [DOI] [PubMed] [Google Scholar]
- Bell S. C., Bowerman A. M., Nixon L. E., Macdonald I. A., Elborn J. S., Shale D. J. Metabolic and inflammatory responses to pulmonary exacerbation in adults with cystic fibrosis. Eur J Clin Invest. 2000 Jun;30(6):553–559. doi: 10.1046/j.1365-2362.2000.00667.x. [DOI] [PubMed] [Google Scholar]
- Benabdeslam H., Garcia I., Bellon G., Gilly R., Revol A. Biochemical assessment of the nutritional status of cystic fibrosis patients treated with pancreatic enzyme extracts. Am J Clin Nutr. 1998 May;67(5):912–918. doi: 10.1093/ajcn/67.5.912. [DOI] [PubMed] [Google Scholar]
- Bussières L., Souberbielle J. C., Pinto G., Adan L., Noel M., Brauner R. The use of insulin-like growth factor 1 reference values for the diagnosis of growth hormone deficiency in prepubertal children. Clin Endocrinol (Oxf) 2000 Jun;52(6):735–739. doi: 10.1046/j.1365-2265.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- Caregaro L., Favaro A., Santonastaso P., Alberino F., Di Pascoli L., Nardi M., Favaro S., Gatta A. Insulin-like growth factor 1 (IGF-1), a nutritional marker in patients with eating disorders. Clin Nutr. 2001 Jun;20(3):251–257. doi: 10.1054/clnu.2001.0397. [DOI] [PubMed] [Google Scholar]
- Checketts S. R., Morrison K. A., Baughman R. D. Nonsteroidal anti-inflammatory-induced pseudoporphyria: is there an alternative drug? Cutis. 1999 Apr;63(4):223–225. [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E., Dickerson R. N., Brown R. O., Hak L. J., MacPhee R. D., Heizer W. D. Use of plasma somatomedin-C/insulin-like growth factor I measurements to monitor the response to nutritional repletion in malnourished patients. Am J Clin Nutr. 1985 Feb;41(2):191–198. doi: 10.1093/ajcn/41.2.191. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E. Role of insulin-like growth factors and growth hormone in reversing catabolic states. Horm Res. 1992;38 (Suppl 2):37–40. doi: 10.1159/000182591. [DOI] [PubMed] [Google Scholar]
- Coolican S. A., Samuel D. S., Ewton D. Z., McWade F. J., Florini J. R. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997 Mar 7;272(10):6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- Corey M., McLaughlin F. J., Williams M., Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41(6):583–591. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Creemers M. C., Chang A., Franssen M. J., Fiselier T. J., van Riel P. L. Pseudoporphyria due to naproxen. A cluster of 3 cases. Scand J Rheumatol. 1995;24(3):185–187. doi: 10.3109/03009749509099314. [DOI] [PubMed] [Google Scholar]
- De Silva B., Banney L., Uttley W., Luqmani R., Schofield O. Pseudoporphyria and nonsteroidal antiinflammatory agents in children with juvenile idiopathic arthritis. Pediatr Dermatol. 2000 Nov-Dec;17(6):480–483. doi: 10.1046/j.1525-1470.2000.01827.x. [DOI] [PubMed] [Google Scholar]
- Donahue S. P., Phillips L. S. Response of IGF-1 to nutritional support in malnourished hospital patients: a possible indicator of short-term changes in nutritional status. Am J Clin Nutr. 1989 Nov;50(5):962–969. doi: 10.1093/ajcn/50.5.962. [DOI] [PubMed] [Google Scholar]
- Eid A. A., Ionescu A. A., Nixon L. S., Lewis-Jenkins V., Matthews S. B., Griffiths T. L., Shale D. J. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001 Oct 15;164(8 Pt 1):1414–1418. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- Gelander L., Blum W. F., Larsson L., Rosberg S., Albertsson-Wikland K. Monthly measurements of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in healthy prepubertal children: characterization and relationship with growth: the 1-year growth study. Pediatr Res. 1999 Mar;45(3):377–383. doi: 10.1203/00006450-199903000-00015. [DOI] [PubMed] [Google Scholar]
- Hayot M., Guillaumont S., Ramonatxo M., Voisin M., Préfaut C. Determinants of the tension-time index of inspiratory muscles in children with cystic fibrosis. Pediatr Pulmonol. 1997 May;23(5):336–343. doi: 10.1002/(sici)1099-0496(199705)23:5<336::aid-ppul5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Ionescu A. A., Chatham K., Davies C. A., Nixon L. S., Enright S., Shale D. J. Inspiratory muscle function and body composition in cystic fibrosis. Am J Respir Crit Care Med. 1998 Oct;158(4):1271–1276. doi: 10.1164/ajrccm.158.4.9710079. [DOI] [PubMed] [Google Scholar]
- Ionescu A. A., Nixon L. S., Evans W. D., Stone M. D., Lewis-Jenkins V., Chatham K., Shale D. J. Bone density, body composition, and inflammatory status in cystic fibrosis. Am J Respir Crit Care Med. 2000 Sep;162(3 Pt 1):789–794. doi: 10.1164/ajrccm.162.3.9910118. [DOI] [PubMed] [Google Scholar]
- Ionescu Alina A., Nixon Lisette S., Luzio Stephen, Lewis-Jenkins Vanessa, Evans William D., Stone Michael D., Owens David R., Routledge Philip A., Shale Dennis J. Pulmonary function, body composition, and protein catabolism in adults with cystic fibrosis. Am J Respir Crit Care Med. 2002 Feb 15;165(4):495–500. doi: 10.1164/ajrccm.165.4.2104065. [DOI] [PubMed] [Google Scholar]
- Krischer J., Scolari F., Kondo-Oestreicher M., Vollenweider-Roten S., Saurat J. H., Pechère M. Pseudoporphyria induced by nabumetone. J Am Acad Dermatol. 1999 Mar;40(3):492–493. doi: 10.1016/s0190-9622(99)70507-4. [DOI] [PubMed] [Google Scholar]
- Laaban J. P., Kouchakji B., Dore M. F., Orvoen-Frija E., David P., Rochemaure J. Nutritional status of patients with chronic obstructive pulmonary disease and acute respiratory failure. Chest. 1993 May;103(5):1362–1368. doi: 10.1378/chest.103.5.1362. [DOI] [PubMed] [Google Scholar]
- Lang C. H., Frost R. A., Ejiofor J., Lacy D. B., McGuinness O. P. Hepatic production and intestinal uptake of IGF-I: response to infection. Am J Physiol. 1998 Dec;275(6 Pt 1):G1291–G1298. doi: 10.1152/ajpgi.1998.275.6.G1291. [DOI] [PubMed] [Google Scholar]
- Laursen E. M., Juul A., Lanng S., Høiby N., Koch C., Müller J., Skakkebaek N. E. Diminished concentrations of insulin-like growth factor I in cystic fibrosis. Arch Dis Child. 1995 Jun;72(6):494–497. doi: 10.1136/adc.72.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen E. M., Lanng S., Rasmussen M. H., Koch C., Skakkebaek N. E., Müller J. Normal spontaneous and stimulated GH levels despite decreased IGF-I concentrations in cystic fibrosis patients. Eur J Endocrinol. 1999 Apr;140(4):315–321. doi: 10.1530/eje.0.1400315. [DOI] [PubMed] [Google Scholar]
- Lebl J., Zahradníková M., Bartosová J., Zemková D., Pechová M., Vávrová V. Insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in cystic fibrosis: a positive effect of antibiotic therapy and hyperalimentation. Acta Paediatr. 2001 Aug;90(8):868–872. [PubMed] [Google Scholar]
- Lewis M. I., Feinberg A. T., Fournier M. IGF-I and/or growth hormone preserve diaphragm fiber size with moderate malnutrition. J Appl Physiol (1985) 1998 Jul;85(1):189–197. doi: 10.1152/jappl.1998.85.1.189. [DOI] [PubMed] [Google Scholar]
- Numata M., Yamamoto H., Kawaguchi Y., Osaka N., Nakayama M., Kubo H., Shigematsu T., Hosoya T. [A study of association between lean body mass and serum insulin-like growth factor-1 in continuous ambulatory peritoneal dialysis patients]. Nihon Jinzo Gakkai Shi. 1999 Jan;41(1):8–13. [PubMed] [Google Scholar]
- O'Hagan A. H., Irvine A. D., Allen G. E., Walsh M. Pseudoporphyria induced by mefenamic acid. Br J Dermatol. 1998 Dec;139(6):1131–1132. doi: 10.1046/j.1365-2133.1998.2576p.x. [DOI] [PubMed] [Google Scholar]
- Ramsey B. W., Farrell P. M., Pencharz P. Nutritional assessment and management in cystic fibrosis: a consensus report. The Consensus Committee. Am J Clin Nutr. 1992 Jan;55(1):108–116. doi: 10.1093/ajcn/55.1.108. [DOI] [PubMed] [Google Scholar]
- Reilly J. J., Edwards C. A., Weaver L. T. Malnutrition in children with cystic fibrosis: the energy-balance equation. J Pediatr Gastroenterol Nutr. 1997 Aug;25(2):127–136. doi: 10.1097/00005176-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Ross R., Miell J., Freeman E., Jones J., Matthews D., Preece M., Buchanan C. Critically ill patients have high basal growth hormone levels with attenuated oscillatory activity associated with low levels of insulin-like growth factor-I. Clin Endocrinol (Oxf) 1991 Jul;35(1):47–54. doi: 10.1111/j.1365-2265.1991.tb03495.x. [DOI] [PubMed] [Google Scholar]
- SHWACHMAN H., KULCZYCKI L. L. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child. 1958 Jul;96(1):6–15. doi: 10.1001/archpedi.1958.02060060008002. [DOI] [PubMed] [Google Scholar]
- Shoup R., Dalsky G., Warner S., Davies M., Connors M., Khan M., Khan F., ZuWallack R. Body composition and health-related quality of life in patients with obstructive airways disease. Eur Respir J. 1997 Jul;10(7):1576–1580. doi: 10.1183/09031936.97.10071576. [DOI] [PubMed] [Google Scholar]
- Singleton J. R., Feldman E. L. Insulin-like growth factor-I in muscle metabolism and myotherapies. Neurobiol Dis. 2001 Aug;8(4):541–554. doi: 10.1006/nbdi.2001.0416. [DOI] [PubMed] [Google Scholar]
- Smith W. J., Underwood L. E., Clemmons D. R. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J Clin Endocrinol Metab. 1995 Feb;80(2):443–449. doi: 10.1210/jcem.80.2.7531712. [DOI] [PubMed] [Google Scholar]
- Svendsen O. L., Haarbo J., Hassager C., Christiansen C. Accuracy of measurements of body composition by dual-energy x-ray absorptiometry in vivo. Am J Clin Nutr. 1993 May;57(5):605–608. doi: 10.1093/ajcn/57.5.605. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Bush A., Thomson A., Oades P. J., Marchant J. L., Bruce-Morgan C., Holly J., Ahmed L., Dunger D. B. Relation between insulin-like growth factor-I, body mass index, and clinical status in cystic fibrosis. Arch Dis Child. 1997 Apr;76(4):304–309. doi: 10.1136/adc.76.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M., Thomson A., Bruce-Morgan C., Ahmed M. L., Watts A., Harris D., Holly J. M., Dunger D. B. The relationship between insulin, IGF-I and weight gain in cystic fibrosis. Clin Endocrinol (Oxf) 1999 Nov;51(5):659–665. doi: 10.1046/j.1365-2265.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Thissen J. P., Ketelslegers J. M., Underwood L. E. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994 Feb;15(1):80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- Thissen J. P., Underwood L. E., Ketelslegers J. M. Regulation of insulin-like growth factor-I in starvation and injury. Nutr Rev. 1999 Jun;57(6):167–176. doi: 10.1111/j.1753-4887.1999.tb06939.x. [DOI] [PubMed] [Google Scholar]
- Wallace C. A., Farrow D., Sherry D. D. Increased risk of facial scars in children taking nonsteroidal antiinflammatory drugs. J Pediatr. 1994 Nov;125(5 Pt 1):819–822. doi: 10.1016/s0022-3476(94)70084-2. [DOI] [PubMed] [Google Scholar]