Abstract
Central core disease (CCD) is a dominantly inherited congenital myopathy allelic to malignant hyperthermia (MH) caused by mutations in the RYR1 gene on chromosome 19q13.1. Eleven individuals with RYR1 mutations are described. Four index cases showed features consistent with a congenital myopathy (hypotonia, delayed motor milestones, and skeletal abnormalities including congenital hip dislocation and scoliosis). All four cases and subsequently seven other family members were found to possess novel mutations in the RYR1 gene. The degree of disability varied from one clinically normal individual, to another who had never achieved independent ambulation (the only patient with a de novo mutation). Four cases showed a mild reduction in vital capacity, repeated nocturnal polysomnography showed hypoxaemia in one case. A variety of muscle biopsy features were found; central cores were absent in the youngest case, and the biopsy specimens from two others were more suggestive of mini-core myopathy. In all cases missense mutations in exons 101, 102, and 103 of the RYR1 gene on were found. Future laboratory diagnosis of suspected cases and family members will be less invasive and more accurate with DNA analysis. Clinicians, especially paediatricians and orthopaedic surgeons, should be aware of this disorder because of the potential risk of MH.
Full Text
The Full Text of this article is available as a PDF (288.1 KB).
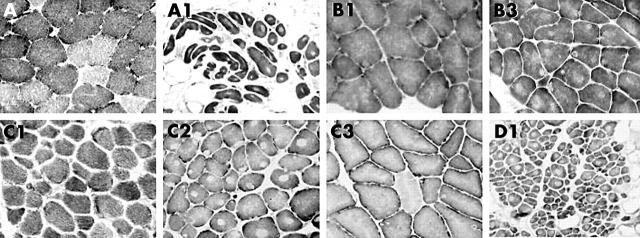
Figure 1 .
Muscle biopsies stained for NADH-TR. A: normal two fibre pattern. A1: type 1 fibre uniformity (all dark fibres), central cores, and increased adipose and connective tissue. B1 (B2 similar): type 1 fibre uniformity with uneven staining and small core-like areas. B3: type 1fibre uniformity and multiple central and eccentric cores. C1: type 1 fibre uniformity. C2: abundant well defined central and eccentric cores with type 1 fibre uniformity. C3: type 1 fibre predominance, uneven stain, and small eccentric cores. D1: type uniformity, with cores in most fibres, a wide variability in fibre size, and excess adipose tissue.
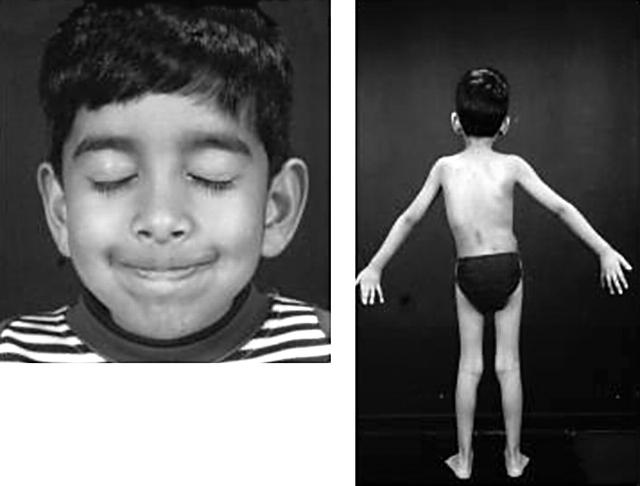
Figure 2 .
Case B1, showing mild facial weakness with an inability to bury the eyelashes and early onset scoliosis.
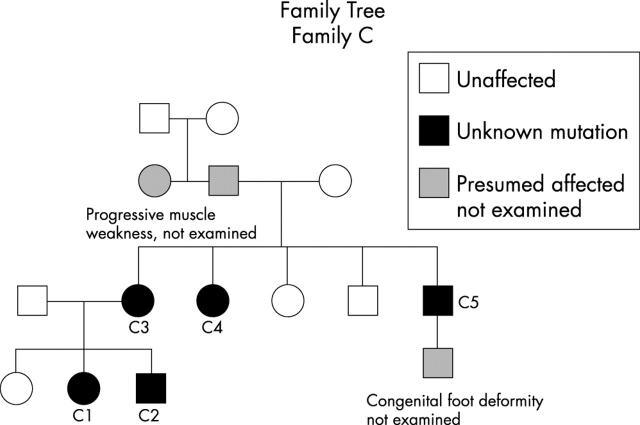
Figure 3 .
Family tree of family 3.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Girard T., Cavagna D., Padovan E., Spagnoli G., Urwyler A., Zorzato F., Treves S. B-lymphocytes from malignant hyperthermia-susceptible patients have an increased sensitivity to skeletal muscle ryanodine receptor activators. J Biol Chem. 2001 Oct 22;276(51):48077–48082. doi: 10.1074/jbc.M107134200. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Miller R. G., Brownell A. K. Central core disease: ultrastructure of the sarcoplasmic reticulum and T-tubules. Muscle Nerve. 1989 Feb;12(2):95–102. doi: 10.1002/mus.880120203. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K., McCarthy T., Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve. 2000 Jan;23(1):4–17. doi: 10.1002/(sici)1097-4598(200001)23:1<4::aid-mus3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- King J. O., Denborough M. A. Malignant hyperpyrexia in Australia and New Zealand. Med J Aust. 1973 Mar 17;1(11):525–528. doi: 10.5694/j.1326-5377.1973.tb110542.x. [DOI] [PubMed] [Google Scholar]
- Kosko J. R., Brandom B. W., Chan K. H. Masseter spasm and malignant hyperthermia: a retrospective review of a hospital-based pediatric otolaryngology practice. Int J Pediatr Otorhinolaryngol. 1992 Jan;23(1):45–50. doi: 10.1016/0165-5876(92)90078-4. [DOI] [PubMed] [Google Scholar]
- Larach M. G. Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group. Anesth Analg. 1989 Oct;69(4):511–515. [PubMed] [Google Scholar]
- Loke J., MacLennan D. H. Malignant hyperthermia and central core disease: disorders of Ca2+ release channels. Am J Med. 1998 May;104(5):470–486. doi: 10.1016/s0002-9343(98)00108-9. [DOI] [PubMed] [Google Scholar]
- MAGEE K. R., SHY G. M. A new congenital non-progressive myopathy. Brain. 1956 Dec;79(4):610–621. doi: 10.1093/brain/79.4.610. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Duff C., Zorzato F., Fujii J., Phillips M., Korneluk R. G., Frodis W., Britt B. A., Worton R. G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990 Feb 8;343(6258):559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- Manzur A. Y., Sewry C. A., Ziprin J., Dubowitz V., Muntoni F. A severe clinical and pathological variant of central core disease with possible autosomal recessive inheritance. Neuromuscul Disord. 1998 Oct;8(7):467–473. doi: 10.1016/s0960-8966(98)00064-9. [DOI] [PubMed] [Google Scholar]
- McCarthy T. V., Quane K. A., Lynch P. J. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum Mutat. 2000;15(5):410–417. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Monnier N., Romero N. B., Lerale J., Landrieu P., Nivoche Y., Fardeau M., Lunardi J. Familial and sporadic forms of central core disease are associated with mutations in the C-terminal domain of the skeletal muscle ryanodine receptor. Hum Mol Genet. 2001 Oct 15;10(22):2581–2592. doi: 10.1093/hmg/10.22.2581. [DOI] [PubMed] [Google Scholar]
- Putaala H., Soininen R., Kilpeläinen P., Wartiovaara J., Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001 Jan 1;10(1):1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- Sewry C. A., Müller C., Davis M., Dwyer J. S. M., Dove J., Evans G., Schröder R., Fürst D., Helliwell T., Laing N. The spectrum of pathology in central core disease. Neuromuscul Disord. 2002 Dec;12(10):930–938. doi: 10.1016/s0960-8966(02)00135-9. [DOI] [PubMed] [Google Scholar]
- Tojo M., Ozawa M., Nonaka I. Central core disease and congenital neuromuscular disease with uniform type 1 fibers in one family. Brain Dev. 2000 Jun;22(4):262–264. doi: 10.1016/s0387-7604(00)00108-x. [DOI] [PubMed] [Google Scholar]