Abstract
Background: A phenylalanine-free amino acid based protein substitute is necessary to provide the major source of protein in phenylketonuria (PKU). Protein substitutes in PKU are usually given as drinks. These are unpalatable and compliance is often poor. Tablets containing a suitable mixture of phenylalanine-free amino acids (Aminogran Food Supplement, UCB) are now available.
Aims: To compare the effectiveness and acceptability of these tablets with conventional protein substitute drinks.
Methods: Twenty one subjects with PKU, aged 8–25 years, participated in a randomised crossover study. During one phase, subjects received at least 40% of their protein substitute requirements from the amino acid tablets and the rest from their usual protein substitute tablets. During the other phase, they received their usual protein substitute. Each period lasted 12 weeks. Blood phenylalanine concentrations were measured at least once every two weeks and other plasma amino acids were measured at the beginning, at crossover, and at the end of the study. The subjects kept a diary of all protein substitute taken.
Results: Compliance appeared to be better with the new tablets than with patients' usual protein substitutes. Ninety per cent (18/20) recorded that they took the tablets as prescribed, compared with 65% (13/20) fully compliant with their usual protein substitute. Moreover, plasma phenyalanine was lower on the amino acid tablets, and the median difference in blood concentrations between the two groups was 46 µmol/l (95% CI 14.8 to 89.0, p = 0.02). Tyrosine increased by a median of 16 µmol/l daily on the amino acid tablets (95% CI 7.1 to 40.5, p = 0.01). Most subjects (70%) preferred incorporating the new tablets into their usual protein substitute regimen.
Conclusions: Amino acid tablets are an effective and relatively popular protein substitute in older children, teenagers, and adults with PKU.
Full Text
The Full Text of this article is available as a PDF (112.9 KB).
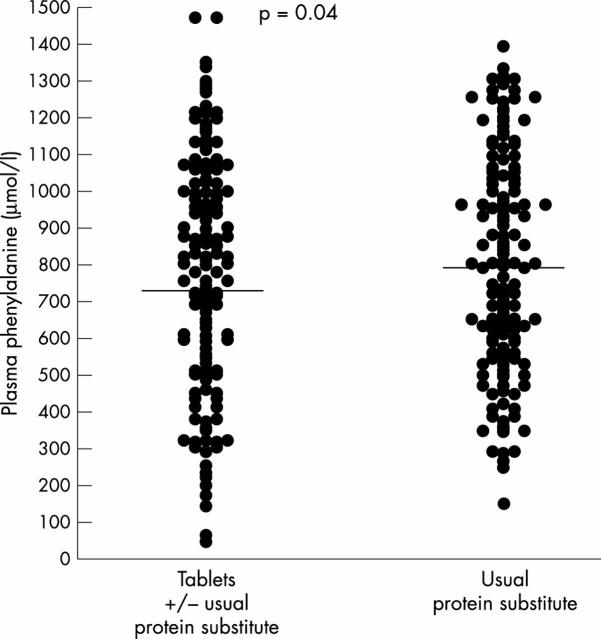
Figure 1.
Chart showing the differences for each subject of the phenylalanine concentration changes under regimen B minus those under regimen A, subgrouped by the order of getting the two regimes. Horizontal line represents mean.
Figure 2.
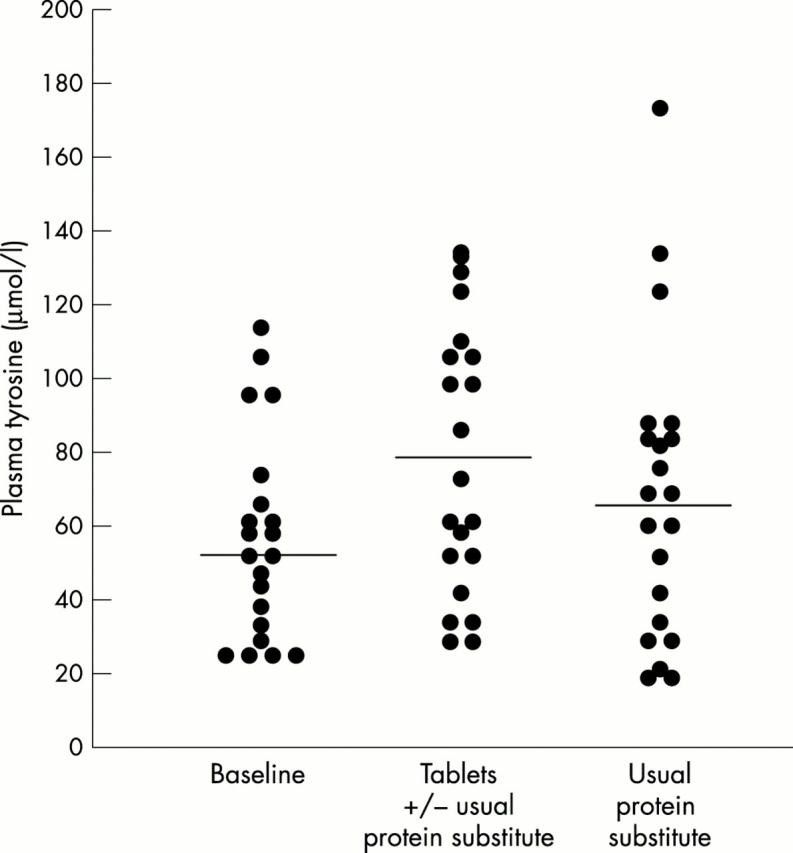
Chart showing the changes for each subject in tyrosine concentration during the periods under regimen A and regimen B. Horizontal line represents mean.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta P. B., Yannicelli S. Protein intake affects phenylalanine requirements and growth of infants with phenylketonuria. Acta Paediatr Suppl. 1994 Dec;407:66–67. doi: 10.1111/j.1651-2227.1994.tb13454.x. [DOI] [PubMed] [Google Scholar]
- Kecskemethy H. H., Lobbregt D., Levy H. L. The use of gelatin capsules for ingestion of formula in dietary treatment of maternal phenylketonuria. J Inherit Metab Dis. 1993;16(1):111–118. doi: 10.1007/BF00711324. [DOI] [PubMed] [Google Scholar]
- MUNRO H. N., THOMSON W. S. Influence of glucose on amino acid metabolism. Metabolism. 1953 Jul;2(4):354–361. [PubMed] [Google Scholar]
- MacDonald A., Rylance G. W., Asplin D., Hall S. K., Booth I. W. Does a single plasma phenylalanine predict quality of control in phenylketonuria? Arch Dis Child. 1998 Feb;78(2):122–126. doi: 10.1136/adc.78.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A., Rylance G., Hall S. K., Asplin D., Booth I. W. Factors affecting the variation in plasma phenylalanine in patients with phenylketonuria on diet. Arch Dis Child. 1996 May;74(5):412–417. doi: 10.1136/adc.74.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt O. E. A new approach to the treatment of phenylketonuria. J Ment Defic Res. 1980 Sep;24(3):203–217. doi: 10.1111/j.1365-2788.1980.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Prince A. P., McMurray M. P., Buist N. R. Treatment products and approaches for phenylketonuria: improved palatability and flexibility demonstrate safety, efficacy and acceptance in US clinical trials. J Inherit Metab Dis. 1997 Aug;20(4):486–498. doi: 10.1023/a:1005337126669. [DOI] [PubMed] [Google Scholar]
- Schulz B., Bremer H. J. Nutrient intake and food consumption of adolescents and young adults with phenylketonuria. Acta Paediatr. 1995 Jul;84(7):743–748. doi: 10.1111/j.1651-2227.1995.tb13748.x. [DOI] [PubMed] [Google Scholar]