Abstract
Aim: To examine the effectiveness of delivery of nebulised colistin by the HaloLite nebuliser compared to the Pari LC Plus in patients with cystic fibrosis.
Methods: Randomised crossover trial of 15 patients aged >6 years. Inhalation of one mega unit of colistin in 3 ml diluent, labelled with technetium-99m DTPA, was used to assess lung deposition. The Pari was nebulised to dryness and one button press of the HaloLite was completed. Following a seven day washout period, patients inhaled colistin twice daily for seven days through the first device. Sputum specimens were analysed for colistin levels and pseudomonas load. This procedure was repeated with the alternative device.
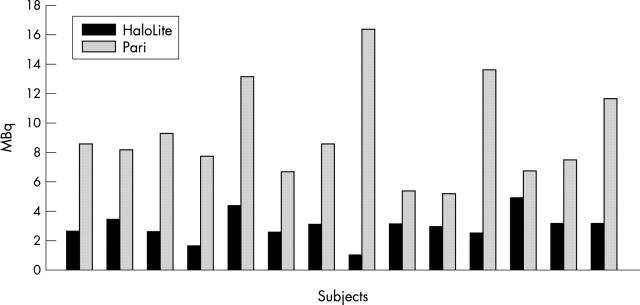
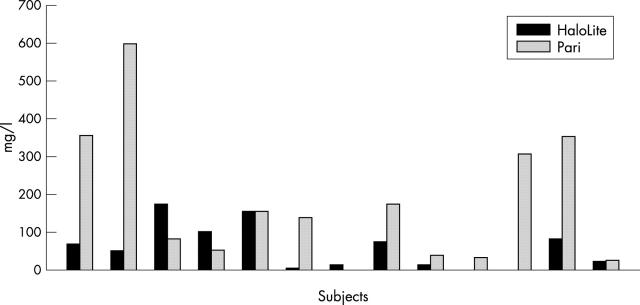
Results: Lung uptake of radiolabelled colistin was significantly higher with the Pari. However, lung uptake calculated as a percentage of the amount of drug used was significantly higher for the HaloLite. Time to nebulise was significantly shorter with the HaloLite. Sputum levels of colistin were higher following use of the Pari; this was close to significance.
Conclusion: The manufacturer's recommended dosages for nebulising antibiotics with a HaloLite result in a lower delivery than patients receive when using a Pari nebuliser. The concept of adaptive aerosol delivery has several theoretical advantages but the recommended doses for the HaloLite need to be modified in order to improve effectiveness.
Full Text
The Full Text of this article is available as a PDF (118.1 KB).
Figure 1 .
Lung deposition of radiolabelled colistin for each subject following use of the HaloLite and Pari LC Plus nebulisers.
Figure 2 .
Colistin levels in sputum at one hour after use of the HaloLite and Pari LC Plus nebulisers.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Dodd M., Bilton D., Webb A. K. Treatment compliance in adults with cystic fibrosis. Thorax. 1994 Feb;49(2):115–120. doi: 10.1136/thx.49.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua H. L., Collis G. G., Le Souëf P. N. Bronchial response to nebulized antibiotics in children with cystic fibrosis. Eur Respir J. 1990 Nov;3(10):1114–1116. [PubMed] [Google Scholar]
- Conway S. P., Pond M. N., Hamnett T., Watson A. Compliance with treatment in adult patients with cystic fibrosis. Thorax. 1996 Jan;51(1):29–33. doi: 10.1136/thx.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham S., Prasad A., Collyer L., Carr S., Lynn I. B., Wallis C. Bronchoconstriction following nebulised colistin in cystic fibrosis. Arch Dis Child. 2001 May;84(5):432–433. doi: 10.1136/adc.84.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd M. E., Abbott J., Maddison J., Moorcroft A. J., Webb A. K. Effect of tonicity of nebulised colistin on chest tightness and pulmonary function in adults with cystic fibrosis. Thorax. 1997 Jul;52(7):656–658. doi: 10.1136/thx.52.7.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson M. E., Penketh A. R., Batten J. C. Aerosol carbenicillin and gentamicin treatment of Pseudomonas aeruginosa infection in patients with cystic fibrosis. Lancet. 1981 Nov 21;2(8256):1137–1139. doi: 10.1016/s0140-6736(81)90588-2. [DOI] [PubMed] [Google Scholar]
- Littlewood J. M., Miller M. G., Ghoneim A. T., Ramsden C. H. Nebulised colomycin for early pseudomonas colonisation in cystic fibrosis. Lancet. 1985 Apr 13;1(8433):865–865. doi: 10.1016/s0140-6736(85)92222-6. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S., Singh M., Cater J. I., Ogston S., Franklin M., Olver R. E. Nebulised antipseudomonal antibiotic therapy in cystic fibrosis: a meta-analysis of benefits and risks. Thorax. 1996 Apr;51(4):364–368. doi: 10.1136/thx.51.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty M. J., Thomas S. H., Gibb D., Page C. J., Harrington C., Duggan C., Nunan T. O., Bateman N. T. Lung deposition of nebulised pentamidine in children. Thorax. 1993 Mar;48(3):220–226. doi: 10.1136/thx.48.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher-Wilmott R. W., Levinsky R. J., Gordon I., Turner M. W., Matthew D. J. Pseudomonas infection, allergy, and cystic fibrosis. Arch Dis Child. 1982 Aug;57(8):582–586. doi: 10.1136/adc.57.8.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touw D. J., Brimicombe R. W., Hodson M. E., Heijerman H. G., Bakker W. Inhalation of antibiotics in cystic fibrosis. Eur Respir J. 1995 Sep;8(9):1594–1604. [PubMed] [Google Scholar]
- Valerius N. H., Koch C., Høiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet. 1991 Sep 21;338(8769):725–726. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]