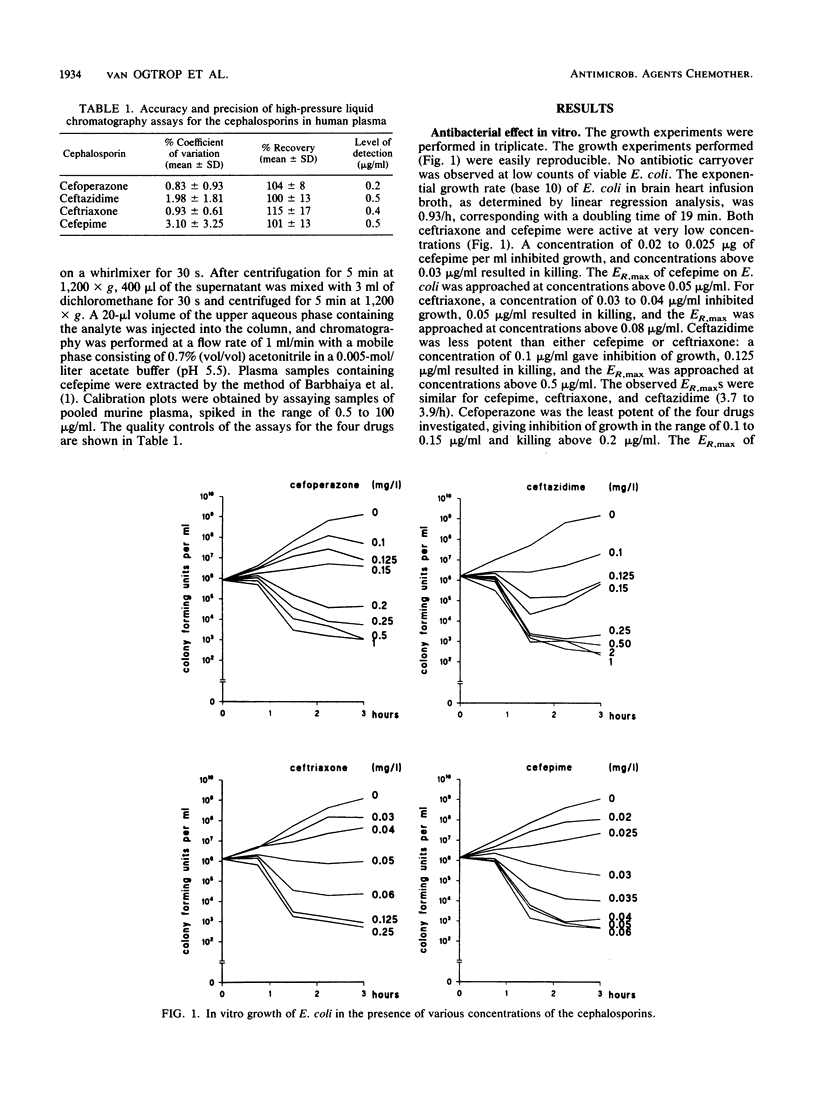
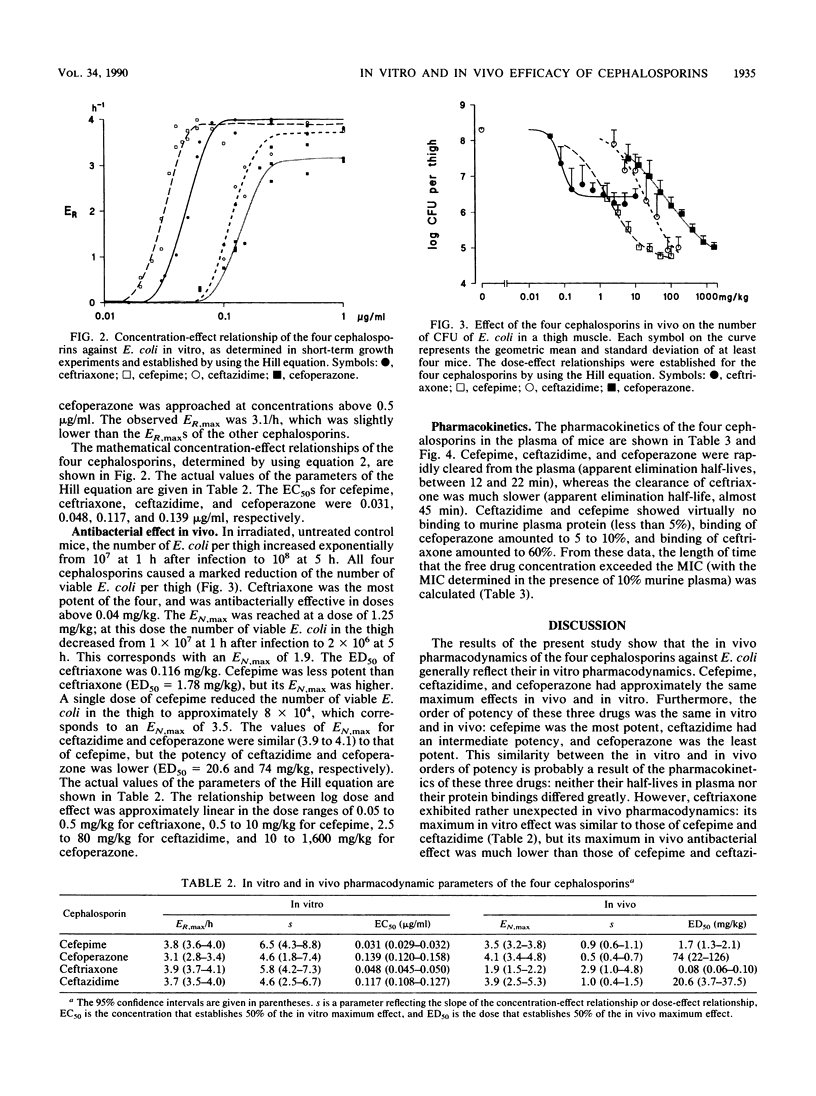
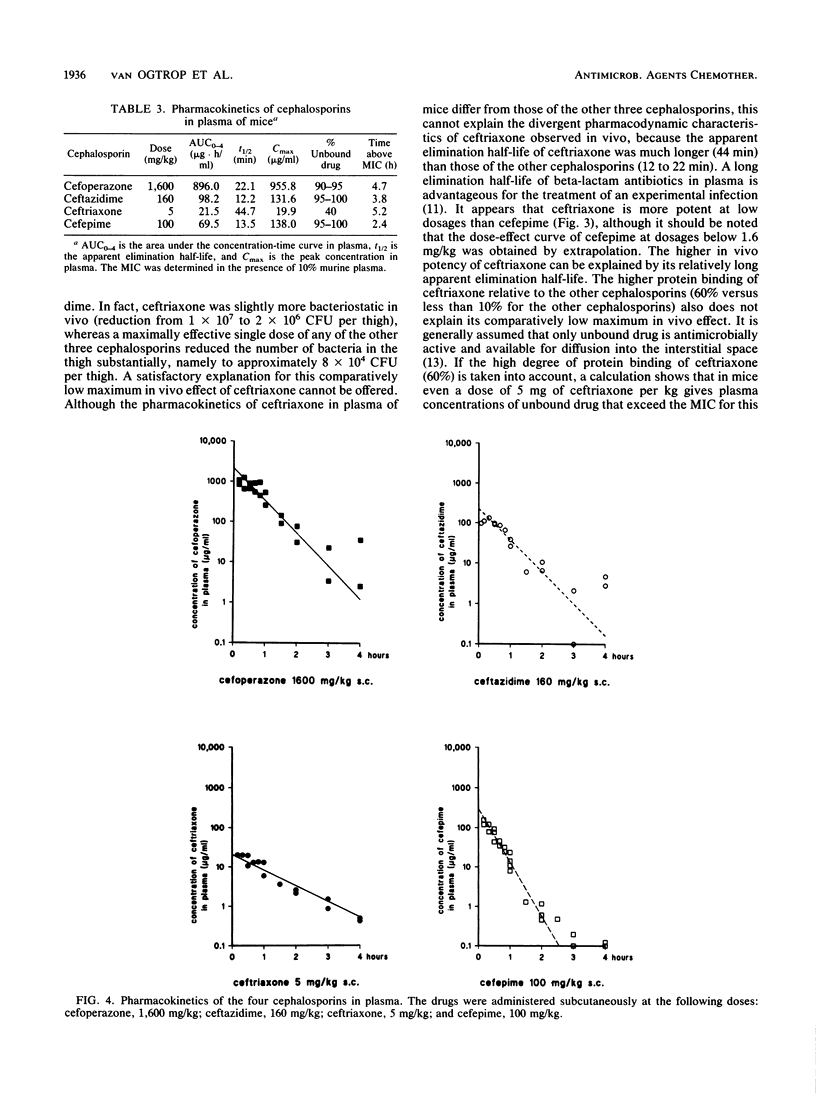
Abstract
A thigh muscle infection induced with Escherichia coli in irradiated mice was used as a model to compare the in vivo pharmacodynamics of the antibacterial effect of four cephalosporins (i.e., cefepime, ceftriaxone, ceftazidime, and cefoperazone) with the in vitro antibacterial pharmacodynamics of these drugs. The following in vitro pharmacodynamic parameters were determined: the maximum effect as a measure for efficacy, the 50% effective concentration as a parameter for potency, and the slope of the concentration-effect relationship. For analysis of the in vivo antibacterial pharmacodynamics, the same parameters were applied for the dose instead of the concentration. For the detection of a relationship between concentration and antibacterial effect in vivo, we determined the pharmacokinetics of the four cephalosporins in the plasma of mice. The results showed that, in general, there is a direct relationship between the in vivo and in vitro pharmacodynamics of these cephalosporins. The maximum effects of cefepime, ceftazidime, and cefoperazone were approximately similar in vivo and in vitro. The sequence of potency of these drugs was, in descending order, cefepime, ceftazidime, and cefoperazone. Ceftriaxone differed from the other three cephalosporins in that it displayed unexpected in vivo pharmacodynamics. Ceftriaxone was just as efficacious as the other three in vitro, but its maximum effect in vivo was much lower. This relatively low maximum effect of ceftriaxone in vivo was not explained by the pharmacokinetic characteristics of the drug. From the present results it can be concluded that the in vitro efficacy of cephalosporins does not necessarily have a predictive value for the in vivo efficacy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Forgue S. T., Shyu W. C., Papp E. A., Pittman K. A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987 Jan;31(1):55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimodt-Møller N., Bentzon M. W., Thomsen V. F. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986 Sep;154(3):511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- Klastersky J. Empiric treatment of infection during granulocytopenia: a comprehensive approach. Infection. 1989 Mar-Apr;17(2):59–64. doi: 10.1007/BF01646877. [DOI] [PubMed] [Google Scholar]
- Kunst M. W., Mattie H. Cefazolin and cephradine: relationship between antibacterial activity in vitro and in mice experimentally infected with Escherichia coli. J Infect Dis. 1978 Apr;137(4):391–402. doi: 10.1093/infdis/137.4.391. [DOI] [PubMed] [Google Scholar]
- Kunst M. W., Mattie H. Cefazolin and cephradine: relationship between serum concentrations and tissue contents in mice. Infection. 1978;6(4):166–170. doi: 10.1007/BF01641906. [DOI] [PubMed] [Google Scholar]
- Mattie H., Hoogeterp J. J., Hermans J. The relation between plasma and tissue concentrations of antibiotics. Description of a method. J Pharmacokinet Biopharm. 1987 Apr;15(2):191–202. doi: 10.1007/BF01062343. [DOI] [PubMed] [Google Scholar]
- Mattie H., van der Voet G. B. The relative potency of amoxycillin and ampicillin in vitro and in vivo. Scand J Infect Dis. 1981;13(4):291–296. doi: 10.3109/inf.1981.13.issue-4.10. [DOI] [PubMed] [Google Scholar]
- McDonald P. J., Wetherall B. L., Pruul H. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev Infect Dis. 1981 Jan-Feb;3(1):38–44. doi: 10.1093/clinids/3.1.38. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Leggett J., Turnidge J., Ebert S., Craig W. A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988 Oct;158(4):831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- Whiting B., Kelman A. W. The modelling of drug response. Clin Sci (Lond) 1980 Nov;59(5):311–315. doi: 10.1042/cs0590311. [DOI] [PubMed] [Google Scholar]
- Wise R. The relevance of pharmacokinetics to in-vitro models: protein binding--does it matter? J Antimicrob Chemother. 1985 Jan;15 (Suppl A):77–83. doi: 10.1093/jac/15.suppl_a.77. [DOI] [PubMed] [Google Scholar]


