Abstract
Aims: To examine the dose-response relation of inhaled fluticasone for both efficacy and adrenal function in children with asthma.
Methods: Systematic review of double blind randomised dose-response studies of fluticasone in children of at least 4 weeks duration. Main outcome measures: FEV1, morning peak expiratory flow, night awakenings, ß agonist use, major exacerbations, 12 or 24 hour urinary cortisol, peak plasma cortisol post-stimulation.
Results: Seven studies of 1733 children with asthma met the inclusion criteria for efficacy. The dose-response curve for each efficacy outcome measure suggested that the response began to plateau between 100 and 200 µg per day with additional efficacy at the 400 µg per day dose shown in one study of severe asthmatics. Five studies of 1096 children with asthma met the inclusion criteria for assessment of adrenal function. The largest placebo controlled study of 437 children reported no difference in 24 hour urinary cortisol between placebo and fluticasone at doses of 100 and 200 µg per day. The non-placebo controlled study of 528 children reported significant suppression of overnight urinary cortisol levels with fluticasone at 400 compared with 200 µg per day.
Conclusions: There is insufficient data to determine the dose-response of fluticasone in children at doses >400 µg per day. The dose-response curve for fluticasone appears to plateau between 100 and 200 µg per day for efficacy. There was additional efficacy at the 400 µg per day dose in children with severe asthma; however there was evidence of adrenal suppression at this dose.
Full Text
The Full Text of this article is available as a PDF (145.5 KB).
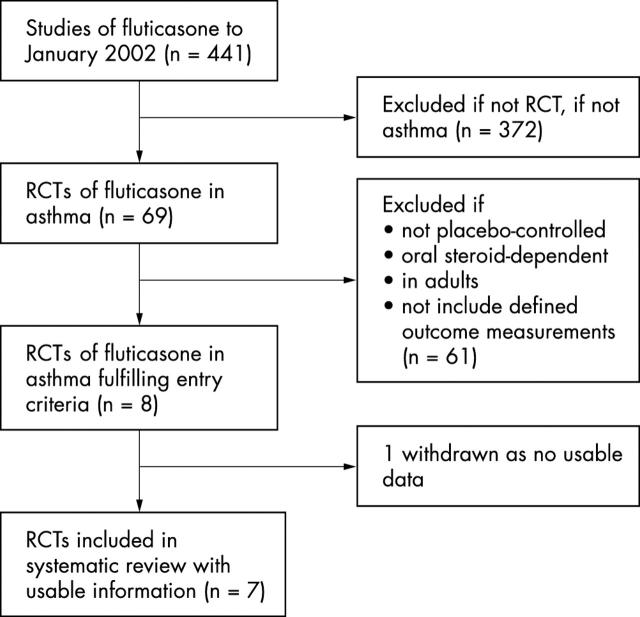
Figure 1.
Process of inclusion of placebo controlled studies in the systematic review.
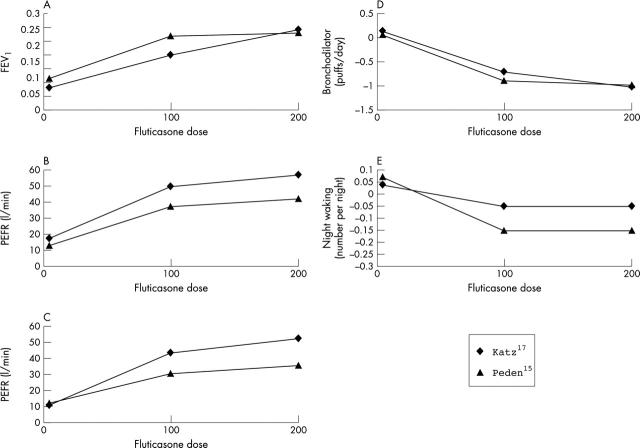
Figure 2.
The change in outcome variable from baseline for (A) FEV1, (B) morning PEF, (C) evening PEF, (D) bronchodilator use, (E) night wakening. Data are taken from Katz et al17 and Peden et al.15
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisgaard H., Damkjaer Nielsen M., Andersen B., Andersen P., Foged N., Fuglsang G., Høst A., Leth C., Pedersen M., Pelck I. Adrenal function in children with bronchial asthma treated with beclomethasone dipropionate or budesonide. J Allergy Clin Immunol. 1988 Jun;81(6):1088–1095. doi: 10.1016/0091-6749(88)90874-3. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Blundell G., Greening A. P., Crompton G. K. Hypothalamo-pituitary-adrenal axis suppression in asthmatics inhaling high dose corticosteroids. Respir Med. 1991 Nov;85(6):501–510. doi: 10.1016/s0954-6111(06)80268-4. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Blundell G., Greening A. P., Crompton G. K. Screening for hypothalamo-pituitary-adrenal axis suppression in asthmatics taking high dose inhaled corticosteroids. Respir Med. 1991 Nov;85(6):511–516. doi: 10.1016/s0954-6111(06)80269-6. [DOI] [PubMed] [Google Scholar]
- Burch W. M. Urine free-cortisol determination. A useful tool in the management of chronic hypoadrenal states. JAMA. 1982 Apr 9;247(14):2002–2004. doi: 10.1001/jama.247.14.2002. [DOI] [PubMed] [Google Scholar]
- Drake A. J., Howells R. J., Shield J. P. H., Prendiville A., Ward P. S., Crowne E. C. Symptomatic adrenal insufficiency presenting with hypoglycaemia in children with asthma receiving high dose inhaled fluticasone propionate. BMJ. 2002 May 4;324(7345):1081–1082. doi: 10.1136/bmj.324.7345.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplantier J. E., Nelson R. P., Jr, Morelli A. R., Good R. A., Kornfeld S. J. Hypothalamic-pituitary-adrenal axis suppression associated with the use of inhaled fluticasone propionate. J Allergy Clin Immunol. 1998 Oct;102(4 Pt 1):699–700. doi: 10.1016/s0091-6749(98)70292-1. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. O., Grol M. H., Bouman K., Stijnen T., Köeter G. H., Kauffman H. F., Gerritsen J. Fluticasone propionate in children with moderate asthma. Am J Respir Crit Care Med. 1996 Oct;154(4 Pt 1):1039–1044. doi: 10.1164/ajrccm.154.4.8887604. [DOI] [PubMed] [Google Scholar]
- Hofstra W. B., Neijens H. J., Duiverman E. J., Kouwenberg J. M., Mulder P. G., Kuethe M. C., Sterk P. J. Dose-responses over time to inhaled fluticasone propionate treatment of exercise- and methacholine-induced bronchoconstriction in children with asthma. Pediatr Pulmonol. 2000 Jun;29(6):415–423. doi: 10.1002/(sici)1099-0496(200006)29:6<415::aid-ppul1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Holt S., Suder A., Weatherall M., Cheng S., Shirtcliffe P., Beasley R. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. BMJ. 2001 Aug 4;323(7307):253–256. doi: 10.1136/bmj.323.7307.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannisto S., Korppi M., Remes K., Voutilainen R. Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. J Clin Endocrinol Metab. 2000 Feb;85(2):652–657. doi: 10.1210/jcem.85.2.6336. [DOI] [PubMed] [Google Scholar]
- Katz Y., Lebas F. X., Medley H. V., Robson R. Fluticasone propionate 50 micrograms BID versus 100 micrograms BID in the treatment of children with persistent asthma. Fluticasone Propionate Study Group. Clin Ther. 1998 May-Jun;20(3):424–437. doi: 10.1016/s0149-2918(98)80053-2. [DOI] [PubMed] [Google Scholar]
- LaForce C. F., Pearlman D. S., Ruff M. E., Silvers W. S., Weinstein S. W., Clements D. S., Brown A., Duke S., Harding S. M., House K. W. Efficacy and safety of dry powder fluticasone propionate in children with persistent asthma. Ann Allergy Asthma Immunol. 2000 Nov;85(5):407–415. doi: 10.1016/S1081-1206(10)62556-2. [DOI] [PubMed] [Google Scholar]
- Li J. T., Ford L. B., Chervinsky P., Weisberg S. C., Kellerman D. J., Faulkner K. G., Herje N. E., Hamedani A., Harding S. M., Shah T. Fluticasone propionate powder and lack of clinically significant effects on hypothalamic-pituitary-adrenal axis and bone mineral density over 2 years in adults with mild asthma. J Allergy Clin Immunol. 1999 Jun;103(6):1062–1068. doi: 10.1016/s0091-6749(99)70180-6. [DOI] [PubMed] [Google Scholar]
- Lipworth B. J., Seckl J. R. Measures for detecting systemic bioactivity with inhaled and intranasal corticosteroids. Thorax. 1997 May;52(5):476–482. doi: 10.1136/thx.52.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie C. A., Weinberg E. G., Tabachnik E., Taylor M., Havnen J., Crescenzi K. A placebo controlled trial of fluticasone propionate in asthmatic children. Eur J Pediatr. 1993 Oct;152(10):856–860. doi: 10.1007/BF02073387. [DOI] [PubMed] [Google Scholar]
- Manjra A. I., Price J., Lenney W., Hughes S., Barnacle H. Efficacy of nebulized fluticasone propionate compared with oral prednisolone in children with an acute exacerbation of asthma. Respir Med. 2000 Dec;94(12):1206–1214. doi: 10.1053/rmed.2000.0952. [DOI] [PubMed] [Google Scholar]
- Patel L., Wales J. K., Kibirige M. S., Massarano A. A., Couriel J. M., Clayton P. E. Symptomatic adrenal insufficiency during inhaled corticosteroid treatment. Arch Dis Child. 2001 Oct;85(4):330–334. doi: 10.1136/adc.85.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman D. S., Noonan M. J., Tashkin D. P., Goldstein M. F., Hamedani A. G., Kellerman D. J., Schaberg A. Comparative efficacy and safety of twice daily fluticasone propionate powder versus placebo in the treatment of moderate asthma. Ann Allergy Asthma Immunol. 1997 Apr;78(4):356–362. doi: 10.1016/S1081-1206(10)63196-1. [DOI] [PubMed] [Google Scholar]
- Peden D. B., Berger W. E., Noonan M. J., Thomas M. R., Hendricks V. L., Hamedani A. G., Mahajan P., House K. W. Inhaled fluticasone propionate delivered by means of two different multidose powder inhalers is effective and safe in a large pediatric population with persistent asthma. J Allergy Clin Immunol. 1998 Jul;102(1):32–38. doi: 10.1016/s0091-6749(98)70052-1. [DOI] [PubMed] [Google Scholar]
- Price J. F., Russell G., Hindmarsh P. C., Weller P., Heaf D. P., Williams J. Growth during one year of treatment with fluticasone propionate or sodium cromoglycate in children with asthma. Pediatr Pulmonol. 1997 Sep;24(3):178–186. doi: 10.1002/(sici)1099-0496(199709)24:3<178::aid-ppul3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Shapiro G., Bronsky E. A., LaForce C. F., Mendelson L., Pearlman D., Schwartz R. H., Szefler S. J. Dose-related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. J Pediatr. 1998 Jun;132(6):976–982. doi: 10.1016/s0022-3476(98)70394-4. [DOI] [PubMed] [Google Scholar]
- Shapiro G., Lumry W., Wolfe J., Given J., White M. V., Woodring A., Baitinger L., House K., Prillaman B., Shah T. Combined salmeterol 50 microg and fluticasone propionate 250 microg in the diskus device for the treatment of asthma. Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):527–534. doi: 10.1164/ajrccm.161.2.9905091. [DOI] [PubMed] [Google Scholar]
- Sorkness C. A., LaForce C., Storms W., Lincourt W. R., Edwards L., Rogenes P. R. Effects of the inhaled corticosteroids fluticasone propionate, triamcinolone acetonide, and flunisolide and oral prednisone on the hypothalamic-pituitary-adrenal axis in adult patients with asthma. Clin Ther. 1999 Feb;21(2):353–367. doi: 10.1016/S0149-2918(00)88292-2. [DOI] [PubMed] [Google Scholar]
- Taylor A. V., Laoprasert N., Zimmerman D., Sachs M. I. Adrenal suppression secondary to inhaled fluticasone propionate. Ann Allergy Asthma Immunol. 1999 Jul;83(1):68–70. doi: 10.1016/S1081-1206(10)63515-6. [DOI] [PubMed] [Google Scholar]
- Todd G. R. G., Acerini C. L., Buck J. J., Murphy N. P., Ross-Russell R., Warner J. T., McCance D. R. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate. Eur Respir J. 2002 Jun;19(6):1207–1209. doi: 10.1183/09031936.02.00274402. [DOI] [PubMed] [Google Scholar]
- Verona E., Petrov D., Cserhati E., Hofman J., Geppe N., Medley H., Hughes S. Fluticasone propionate in asthma: a long term dose comparison study. Arch Dis Child. 2003 Jun;88(6):503–509. doi: 10.1136/adc.88.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. J., Postma D. S., Arends L. R., de Vries T. W., Duiverman E. J., Brand P. L. One-year treatment with different dosing schedules of fluticasone propionate in childhood asthma. Effects on hyperresponsiveness, lung function, and height. Am J Respir Crit Care Med. 2001 Dec 1;164(11):2073–2077. doi: 10.1164/ajrccm.164.11.2103075. [DOI] [PubMed] [Google Scholar]
- Wasserman S. I., Gross G. N., Schoenwetter W. F., Munk Z. M., Kral K. M., Schaberg A., Kellerman D. J. A 12-week dose-ranging study of fluticasone propionate powder in the treatment of asthma. J Asthma. 1996;33(4):265–274. doi: 10.3109/02770909609055367. [DOI] [PubMed] [Google Scholar]
- Wong J. Y. W., Zacharin M. R., Hocking N., Robinson P. J. Growth and adrenal suppression in asthmatic children on moderate to high doses of fluticasone propionate. J Paediatr Child Health. 2002 Feb;38(1):59–62. doi: 10.1046/j.1440-1754.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- de Benedictis F. M., Teper A., Green R. J., Boner A. L., Williams L., Medley H., International Study Group Effects of 2 inhaled corticosteroids on growth: results of a randomized controlled trial. Arch Pediatr Adolesc Med. 2001 Nov;155(11):1248–1254. doi: 10.1001/archpedi.155.11.1248. [DOI] [PubMed] [Google Scholar]