Abstract
Background: Background: Ceftriaxone, a third generation cephalosporin, is widely used for treating infection during childhood. The kidneys eliminate approximately 33–67% of this agent, and the remainder is eliminated via the biliary system. Ceftriaxone may bind with calcium ions and form insoluble precipitate leading to biliary pseudolithiasis. The aim of this study was to assess whether ceftriaxone associated nephrolithiasis develops by the same mechanism, and whether this condition is dose related.
Methods: The study involved 51 children with various infections. Of these, 24 were hospitalized with severe infection and received 100 mg/kg/day ceftriaxone divided into two equal intravenous doses. The other 27 patients received a single daily intramuscular injection of 50 mg/kg/day. Serum and urine parameters were evaluated before and after treatment, and abdominal ultrasonographic examinations were also carried out before and after treatment.
Results: Serum urea, creatinine, and calcium levels were normal in all patients before and after treatment. Post-treatment ultrasound identified nephrolithiasis in four (7.8%) of the 51 subjects. The stones were all of small size (2 mm). Comparison of the groups with and without nephrolithiasis revealed no significant differences with respect to age, sex distribution, duration of treatment, or dose/route of administration of ceftriaxone. The renal stones disappeared spontaneously in three of the four cases, but were still present in one patient 7 months after ceftriaxone treatment.
Conclusions: Conclusions: The study showed that children taking a 7 day course of normal or high dose ceftriaxone may develop small sized asymptomatic renal stones. The overall incidence of nephrolithiasis in this study was 7.8%.
Full Text
The Full Text of this article is available as a PDF (124.5 KB).
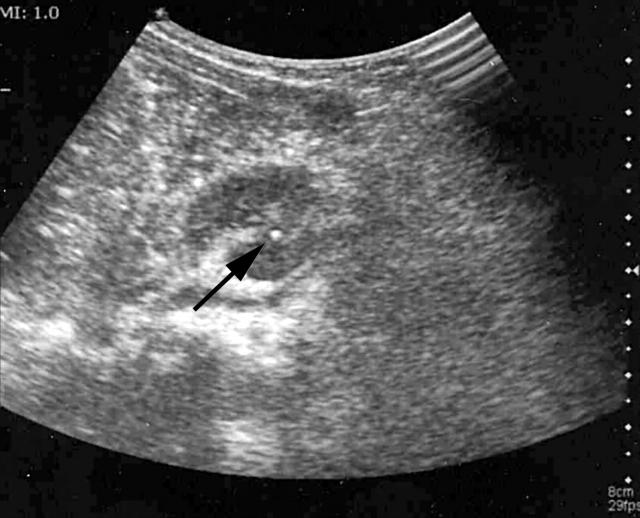
Figure 1.
Right kidney sonogram, the white arrow pointing to the calculi in the lower calyx.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnet J. P., Abid L., Dabhar A., Lévy A., Soulier Y., Blangy S. Early biliary pseudolithiasis during ceftriaxone therapy for acute pyelonephritis in children: a prospective study in 34 children. Eur J Pediatr Surg. 2000 Dec;10(6):368–371. doi: 10.1055/s-2008-1072393. [DOI] [PubMed] [Google Scholar]
- Cochat P., Cochat N., Jouvenet M., Floret D., Wright C., Martin X., Vallon J. J., David L. Ceftriaxone-associated nephrolithiasis. Nephrol Dial Transplant. 1990;5(11):974–976. doi: 10.1093/ndt/5.11.974. [DOI] [PubMed] [Google Scholar]
- Dulac Y., Bouissou F., Azéma C., Barthe P., Baunin C., Normand-Gottis M. Anurie par lithiase urinaire à la ceftriaxone chez un enfant de 6 ans. Presse Med. 1995 May 27;24(19):916–916. [PubMed] [Google Scholar]
- Fehér G., Benczik A., Szabó E., Réthy I., Berényi M. Vesehomok-képzdés és veseelégtelenség ceftriaxon mellékhatásaként. Orv Hetil. 1999 Apr 4;140(14):769–771. [PubMed] [Google Scholar]
- Grasberger H., Otto B., Loeschke K. Ceftriaxone-associated nephrolithiasis. Ann Pharmacother. 2000 Sep;34(9):1076–1077. doi: 10.1345/aph.19363. [DOI] [PubMed] [Google Scholar]
- King W., 3rd, Kimme-Smith C., Winter J. Renal stone shadowing: an investigation of contributing factors. Radiology. 1985 Jan;154(1):191–196. doi: 10.1148/radiology.154.1.3880605. [DOI] [PubMed] [Google Scholar]
- Palanduz A., Yalçin I., Tonguç E., Güler N., Oneş U., Salman N., Somer A. Sonographic assessment of ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound. 2000 May;28(4):166–168. doi: 10.1002/(sici)1097-0096(200005)28:4<166::aid-jcu2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Park H. Z., Lee S. P., Schy A. L. Ceftriaxone-associated gallbladder sludge. Identification of calcium-ceftriaxone salt as a major component of gallbladder precipitate. Gastroenterology. 1991 Jun;100(6):1665–1670. doi: 10.1016/0016-5085(91)90667-a. [DOI] [PubMed] [Google Scholar]
- Prince Jeffrey S., Senac Melvin O., Jr Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis in a child. Pediatr Radiol. 2003 Jun 26;33(9):648–651. doi: 10.1007/s00247-003-0963-0. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Wedgwood-Krucko J., Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet. 1988 Dec 17;2(8625):1411–1413. doi: 10.1016/s0140-6736(88)90596-x. [DOI] [PubMed] [Google Scholar]
- Shiffman M. L., Keith F. B., Moore E. W. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility. Gastroenterology. 1990 Dec;99(6):1772–1778. doi: 10.1016/0016-5085(90)90486-k. [DOI] [PubMed] [Google Scholar]
- Xia Y., Lambert K. J., Schteingart C. D., GU J. J., Hofmann A. F. Concentrative biliary secretion of ceftriaxone. Inhibition of lipid secretion and precipitation of calcium ceftriaxone in bile. Gastroenterology. 1990 Aug;99(2):454–465. doi: 10.1016/0016-5085(90)91029-6. [DOI] [PubMed] [Google Scholar]
- de Moor R. A., Egberts A. C., Schröder C. H. Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis. Eur J Pediatr. 1999 Dec;158(12):975–977. doi: 10.1007/s004310051261. [DOI] [PubMed] [Google Scholar]