Abstract
Objective: To identify factors, particularly the growth hormone (GH) provocation test result, affecting growth response to GH treatment in children with GH deficiency (GHD).
Subjects: A total of 337 prepubertal GHD patients aged <10 years from the UK Pharmacia KIGS database (GH response to provocation test <20 mU/l).
Outcome measure: Annual change in height standard deviation score (SDS) (revised UK reference) in the first and second years of treatment.
Results: Height increased by 0.74 SDS units (SD 0.39) in the first year of treatment and 0.37 units (SD 0.27) in the second. Adjusting for age, height, weight, midparent height, and injection frequency, the strongest predictor of first year growth response was the GH provocation test result; halving the result predicted an extra height increment of 0.09 units (p<0.0001). It predicted the second year response less well (p<0.0002) and after adjusting for the first year response was not predictive at all.
Conclusions: Among patients referred for possible GHD, the GH provocation test, though not a gold standard for diagnosis, is a valuable predictor of growth response in the first year of treatment. A year's treatment is recommended for cases with a marginal provocation test result, with the option to continue treatment if the response is adequate. The value of unified protocols for single or repeated provocation tests needs to be assessed.
Full Text
The Full Text of this article is available as a PDF (90.0 KB).
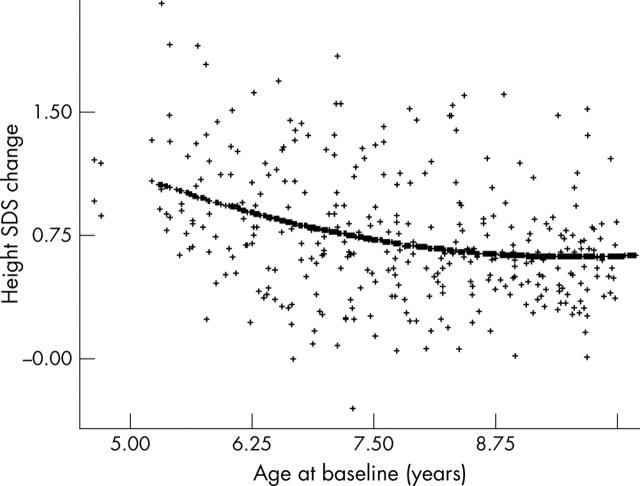
Figure 1.
The curvilinear relation between height SDS change and age in the first year of GH treatment.
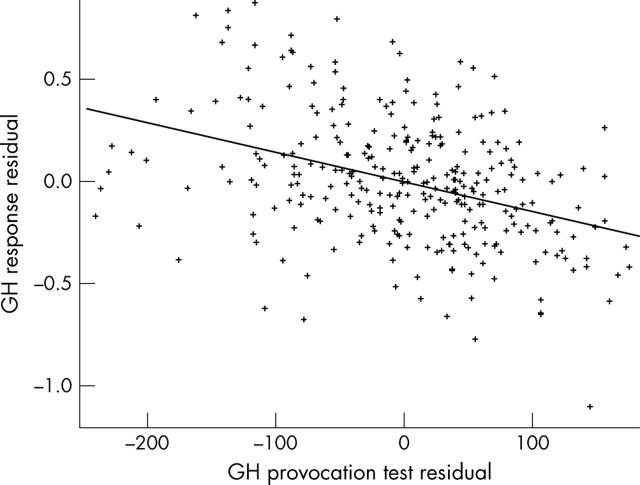
Figure 2.
The relation between height SDS change in the first year of GH treatment and maximum GH peak (%), each expressed as residuals adjusted for the other factors in the model of table 3.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey B. J. Monitoring the heights of prepubertal children. Ann Hum Biol. 1994 Jan-Feb;21(1):1–11. doi: 10.1080/03014469400003032. [DOI] [PubMed] [Google Scholar]
- Bright G. M., Julius J. R., Lima J., Blethen S. L. Growth hormone stimulation test results as predictors of recombinant human growth hormone treatment outcomes: preliminary analysis of the national cooperative growth study database. Pediatrics. 1999 Oct;104(4 Pt 2):1028–1031. [PubMed] [Google Scholar]
- Cole T. J. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med. 2000 Nov 30;19(22):3109–3125. doi: 10.1002/1097-0258(20001130)19:22<3109::aid-sim558>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Darendeliler F., Hindmarsh P. C., Brook C. G. Dose-response curves for treatment with biosynthetic human growth hormone. J Endocrinol. 1990 May;125(2):311–316. doi: 10.1677/joe.0.1250311. [DOI] [PubMed] [Google Scholar]
- Freeman J. V., Cole T. J., Chinn S., Jones P. R., White E. M., Preece M. A. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995 Jul;73(1):17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarsh P. C., Cole T. J. Evidence-based growth hormone therapy prediction models. J Pediatr Endocrinol Metab. 2000;13 (Suppl 6):1359–1364. doi: 10.1515/jpem-2000-s608. [DOI] [PubMed] [Google Scholar]
- Hindmarsh P. C., Smith P. J., Pringle P. J., Brook C. G. The relationship between the response to growth hormone therapy and pre-treatment growth hormone secretory status. Clin Endocrinol (Oxf) 1988 May;28(5):559–563. doi: 10.1111/j.1365-2265.1988.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Russell-Fraser T., Brook C. G., Cotes P. M., Farquhar J. W., Parkin J. M., Preece M. A., Snodgrass G. J., Mason A. S., Tanner J. M. Experience with human growth hormone in Great Britain: the report of the MRC Working Party. Clin Endocrinol (Oxf) 1979 Jul;11(1):15–38. doi: 10.1111/j.1365-2265.1979.tb03043.x. [DOI] [PubMed] [Google Scholar]
- Mitchell H., Dattani M. T., Nanduri V., Hindmarsh P. C., Preece M. A., Brook C. G. Failure of IGF-I and IGFBP-3 to diagnose growth hormone insufficiency. Arch Dis Child. 1999 May;80(5):443–447. doi: 10.1136/adc.80.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece M. A., Freeman J. V., Cole T. J. Sex differences in weight in infancy. Published centile charts for weights have been updated. BMJ. 1996 Dec 7;313(7070):1486–1486. doi: 10.1136/bmj.313.7070.1486a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radetti Giorgio, Buzi Fabio, Cassar Walburga, Paganini Claudio, Stacul Elisabetta, Maghnie Mohamad. Growth hormone secretory pattern and response to treatment in children with short stature followed to adult height. Clin Endocrinol (Oxf) 2003 Jul;59(1):27–33. doi: 10.1046/j.1365-2265.2003.01773.x. [DOI] [PubMed] [Google Scholar]
- Ranke M. B., Guilbaud O. Growth response in prepubertal children with idiopathic growth hormone deficiency during the first year of treatment with human growth hormone. Analysis of the Kabi International Growth Study. Acta Paediatr Scand Suppl. 1990;370:122–130. doi: 10.1111/j.1651-2227.1990.tb11689.x. [DOI] [PubMed] [Google Scholar]
- Ranke M. B., Lindberg A., Chatelain P., Wilton P., Cutfield W., Albertsson-Wikland K., Price D. A. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999 Apr;84(4):1174–1183. doi: 10.1210/jcem.84.4.5634. [DOI] [PubMed] [Google Scholar]
- Rogol Alan D., Blethen Sandra L., Sy Judy P., Veldhuis Johannes D. Do growth hormone (GH) serial sampling, insulin-like growth factor-I (IGF-I) or auxological measurements have an advantage over GH stimulation testing in predicting the linear growth response to GH therapy? Clin Endocrinol (Oxf) 2003 Feb;58(2):229–237. doi: 10.1046/j.1365-2265.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H., Hughes P. C., Vince F. P. Effect of human growth hormone treatment for 1 to 7 years on growth of 100 children, with growth hormone deficiency, low birthweight, inherited smallness, Turner's syndrome, and other complaints. Arch Dis Child. 1971 Dec;46(250):745–782. doi: 10.1136/adc.46.250.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann V., Buckler J. M., Kibirige M. S., Price D. A., Shalet S. M., Wales J. K., Addison M. G., Gill M. S., Whatmore A. J., Clayton P. E. Biochemical tests in the diagnosis of childhood growth hormone deficiency. J Clin Endocrinol Metab. 1997 Feb;82(2):531–535. doi: 10.1210/jcem.82.2.3750. [DOI] [PubMed] [Google Scholar]
- Wit J. M., Faber J. A., Van den Brande J. L. Growth response to human growth hormone treatment in children with partial and total growth hormone deficiency. Acta Paediatr Scand. 1986 Sep;75(5):767–773. doi: 10.1111/j.1651-2227.1986.tb10288.x. [DOI] [PubMed] [Google Scholar]