Abstract
Background: Helicobacter pylori is one of the commonest causes of chronic infection of mankind, yet the natural history of acute infection is poorly understood. Some studies suggest that gastric colonisation with H pylori is associated with suboptimal nutrition and growth in childhood.
Aims: To describe the clinical features of early H pylori colonisation and assess its role in the development of infant malnutrition and growth faltering.
Methods: Two consecutive prospective longitudinal cohort studies were conducted at the Medical Research Council Laboratories in a rural community in The Gambia, West Africa. The first birth cohort of 125 infants was followed by a second of 65 children from the same community. H pylori colonisation was detected by sequential 13C urea breath tests, and infant growth was monitored by serial measurements.
Results: Children with early H pylori colonisation became significantly lighter, shorter, and thinner than their peers in late infancy. The association was found in both cohorts. No socioeconomic or demographic confounding variables were identified to explain this, and the weight deficit was no longer detectable when the children were aged 5–8 years.
Conclusions: Results suggest that H pylori colonisation in early infancy predisposes to the development of malnutrition and growth faltering, although the effect did not persist into later childhood.
Full Text
The Full Text of this article is available as a PDF (147.8 KB).
Figure 1.
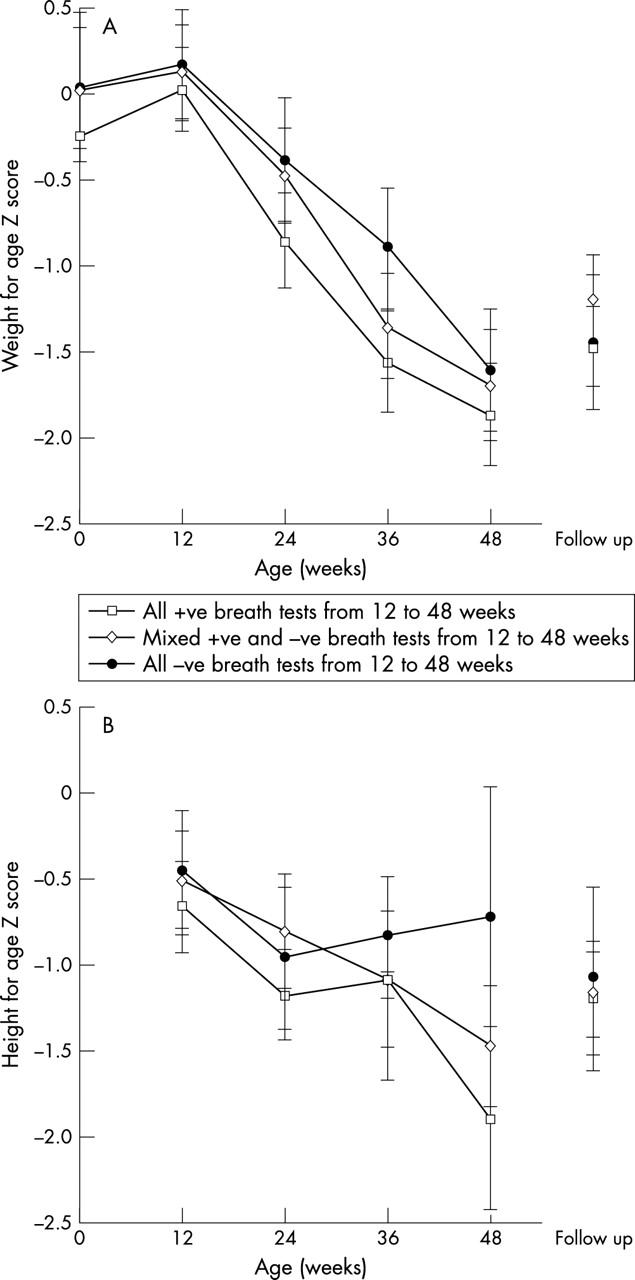
Mean (95% CI) weight-for-age and height-for-age Z scores (relative to NCHS values) for children up to age 1 year from cohort 1. Children are divided into three groups: those with consecutive positive urea breath tests from age 3 months; those with consecutive negative breath tests throughout infancy; and the remaining children with mixed results. Children in the first cohort were studied at 12 week intervals.
Figure 2.
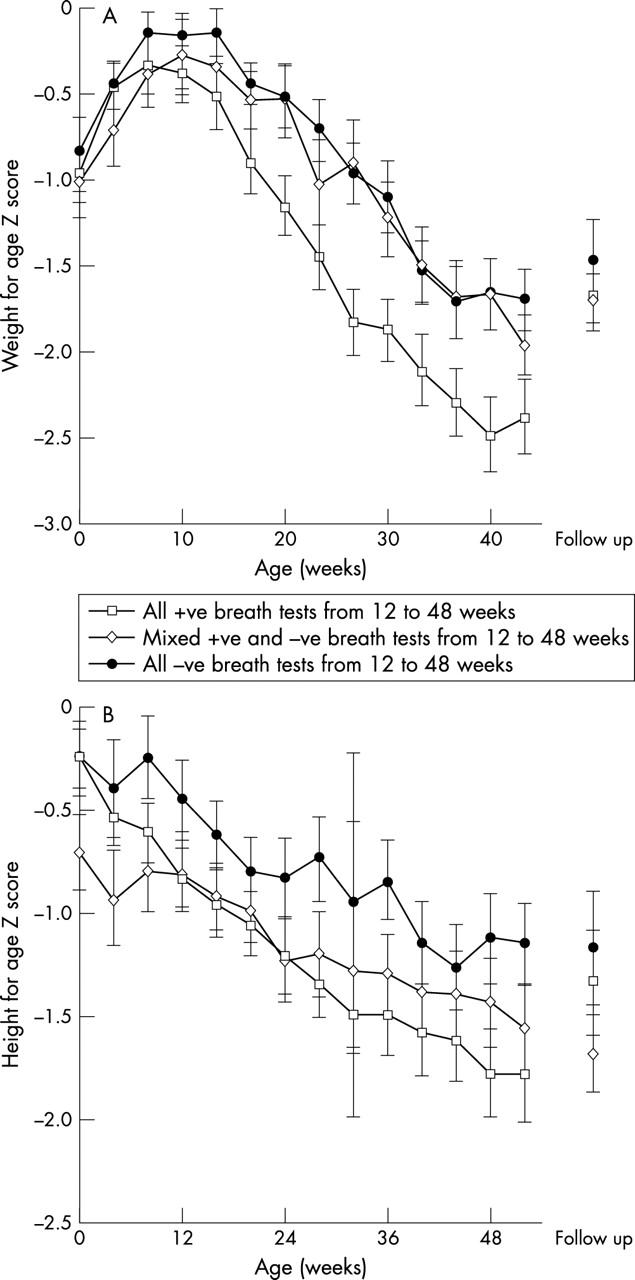
Mean weight-for-age and height-for-age Z scores (relative to NCHS values) for children up to age 1 year from cohort 2. Children are divided into three groups: those with consecutive positive urea breath tests from age 3 months; those with consecutive negative breath tests throughout infancy; and the remaining children with mixed results. Children in the second cohort were studied at four week intervals.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büyükgebiz A., Dündar B., Böber E., Büyükgebiz B. Helicobacter pylori infection in children with constitutional delay of growth and puberty. J Pediatr Endocrinol Metab. 2001 May;14(5):549–551. doi: 10.1515/jpem.2001.14.5.549. [DOI] [PubMed] [Google Scholar]
- Campbell D. I., Warren B. F., Thomas J. E., Figura N., Telford J. L., Sullivan P. B. The African enigma: low prevalence of gastric atrophy, high prevalence of chronic inflammation in West African adults and children. Helicobacter. 2001 Dec;6(4):263–267. doi: 10.1046/j.1083-4389.2001.00047.x. [DOI] [PubMed] [Google Scholar]
- Clemens J., Albert M. J., Rao M., Qadri F., Huda S., Kay B., van Loon F. P., Sack D., Pradhan B. A., Sack R. B. Impact of infection by Helicobacter pylori on the risk and severity of endemic cholera. J Infect Dis. 1995 Jun;171(6):1653–1656. doi: 10.1093/infdis/171.6.1653. [DOI] [PubMed] [Google Scholar]
- Cole T. J. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990 Jan;44(1):45–60. [PubMed] [Google Scholar]
- Dale A., Thomas J. E., Darboe M. K., Coward W. A., Harding M., Weaver L. T. Helicobacter pylori infection, gastric acid secretion, and infant growth. J Pediatr Gastroenterol Nutr. 1998 Apr;26(4):393–397. doi: 10.1097/00005176-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Goodman K. J., Correa P., Tenganá Aux H. J., Ramírez H., DeLany J. P., Guerrero Pepinosa O., López Quiñones M., Collazos Parra T. Helicobacter pylori infection in the Colombian Andes: a population-based study of transmission pathways. Am J Epidemiol. 1996 Aug 1;144(3):290–299. doi: 10.1093/oxfordjournals.aje.a008924. [DOI] [PubMed] [Google Scholar]
- Kehrt R., Becker M., Brösicke H., Krüger N., Helge H. Prevalence of Helicobacter pylori infection in Nicaraguan children with persistent diarrhea, diagnosed by the 13C-urea breath test. J Pediatr Gastroenterol Nutr. 1997 Jul;25(1):84–88. doi: 10.1097/00005176-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Kersulyte D., Mukhopadhyay A. K., Velapatiño B., Su W., Pan Z., Garcia C., Hernandez V., Valdez Y., Mistry R. S., Gilman R. H. Differences in genotypes of Helicobacter pylori from different human populations. J Bacteriol. 2000 Jun;182(11):3210–3218. doi: 10.1128/jb.182.11.3210-3218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaty Hoda M., Haveman Teresa, Graham David Y., Fraley J. Kennard. Helicobacter pylori infection in asymptomatic children: impact of epidemiologic factors on accuracy of diagnostic tests. J Pediatr Gastroenterol Nutr. 2002 Jul;35(1):59–63. doi: 10.1097/00005176-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Helicobacter pylori. Am J Gastroenterol. 1994 Aug;89(8 Suppl):S116–S128. [PubMed] [Google Scholar]
- Oderda G., Palli D., Saieva C., Chiorboli E., Bona G. Short stature and Helicobacter pylori infection in italian children: prospective multicentre hospital based case-control study. The Italian Study Group on Short Stature and H pylori. BMJ. 1998 Aug 22;317(7157):514–515. doi: 10.1136/bmj.317.7157.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro Douglas J., Taylor David N., Gilman Robert H., Cabrera Lilia, Parsonnet Julie. Growth slowing after acute Helicobacter pylori infection is age-dependent. J Pediatr Gastroenterol Nutr. 2002 Oct;35(4):522–526. doi: 10.1097/00005176-200210000-00012. [DOI] [PubMed] [Google Scholar]
- Patel P., Mendall M. A., Khulusi S., Northfield T. C., Strachan D. P. Helicobacter pylori infection in childhood: risk factors and effect on growth. BMJ. 1994 Oct 29;309(6962):1119–1123. doi: 10.1136/bmj.309.6962.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri F., Pastore M., Leandro G., Clemente R., Ghoos Y., Peeters M., Annese V., Quitadamo M., Latiano A., Rutgeerts P. Helicobacter pylori infection and growth delay in older children. Arch Dis Child. 1997 Jul;77(1):46–49. doi: 10.1136/adc.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskitt E. M., Cole T. J., Whitehead R. G. Less diarrhoea but no change in growth: 15 years' data from three Gambian villages. Arch Dis Child. 1999 Feb;80(2):115–120. doi: 10.1136/adc.80.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M., Drumm B. Clinical significance of Helicobacter infection in children. Br Med Bull. 1998;54(1):95–103. doi: 10.1093/oxfordjournals.bmb.a011685. [DOI] [PubMed] [Google Scholar]
- Sarker S. A., Rahman M. M., Mahalanabis D., Bardhan P. K., Hildebrand P., Beglinger C., Gyr K. Prevalence of Helicobacter pylori infection in infants and family contacts in a poor Bangladesh community. Dig Dis Sci. 1995 Dec;40(12):2669–2672. doi: 10.1007/BF02220458. [DOI] [PubMed] [Google Scholar]
- Sullivan P. B., Thomas J. E., Wight D. G., Neale G., Eastham E. J., Corrah T., Lloyd-Evans N., Greenwood B. M. Helicobacter pylori in Gambian children with chronic diarrhoea and malnutrition. Arch Dis Child. 1990 Feb;65(2):189–191. doi: 10.1136/adc.65.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. E., Dale A., Harding M., Coward W. A., Cole T. J., Sullivan P. B., Campbell D. I., Warren B. F., Weaver L. T. Interpreting the 13C-urea breath test among a large population of young children from a developing country. Pediatr Res. 1999 Aug;46(2):147–151. doi: 10.1203/00006450-199908000-00003. [DOI] [PubMed] [Google Scholar]
- Thomas J. E., Dale A., Harding M., Coward W. A., Cole T. J., Weaver L. T. Helicobacter pylori colonization in early life. Pediatr Res. 1999 Feb;45(2):218–223. doi: 10.1203/00006450-199902000-00010. [DOI] [PubMed] [Google Scholar]
- Torres J., Pérez-Pérez G., Goodman K. J., Atherton J. C., Gold B. D., Harris P. R., la Garza A. M., Guarner J., Muñoz O. A comprehensive review of the natural history of Helicobacter pylori infection in children. Arch Med Res. 2000 Sep-Oct;31(5):431–469. doi: 10.1016/s0188-4409(00)00099-0. [DOI] [PubMed] [Google Scholar]
- Weaver L. T., Beckerleg S. Is health a sustainable state? A village study in The Gambia. Lancet. 1993 May 22;341(8856):1327–1330. doi: 10.1016/0140-6736(93)90827-4. [DOI] [PubMed] [Google Scholar]
- Webb P. M., Knight T., Greaves S., Wilson A., Newell D. G., Elder J., Forman D. Relation between infection with Helicobacter pylori and living conditions in childhood: evidence for person to person transmission in early life. BMJ. 1994 Mar 19;308(6931):750–753. doi: 10.1136/bmj.308.6931.750. [DOI] [PMC free article] [PubMed] [Google Scholar]


