Abstract
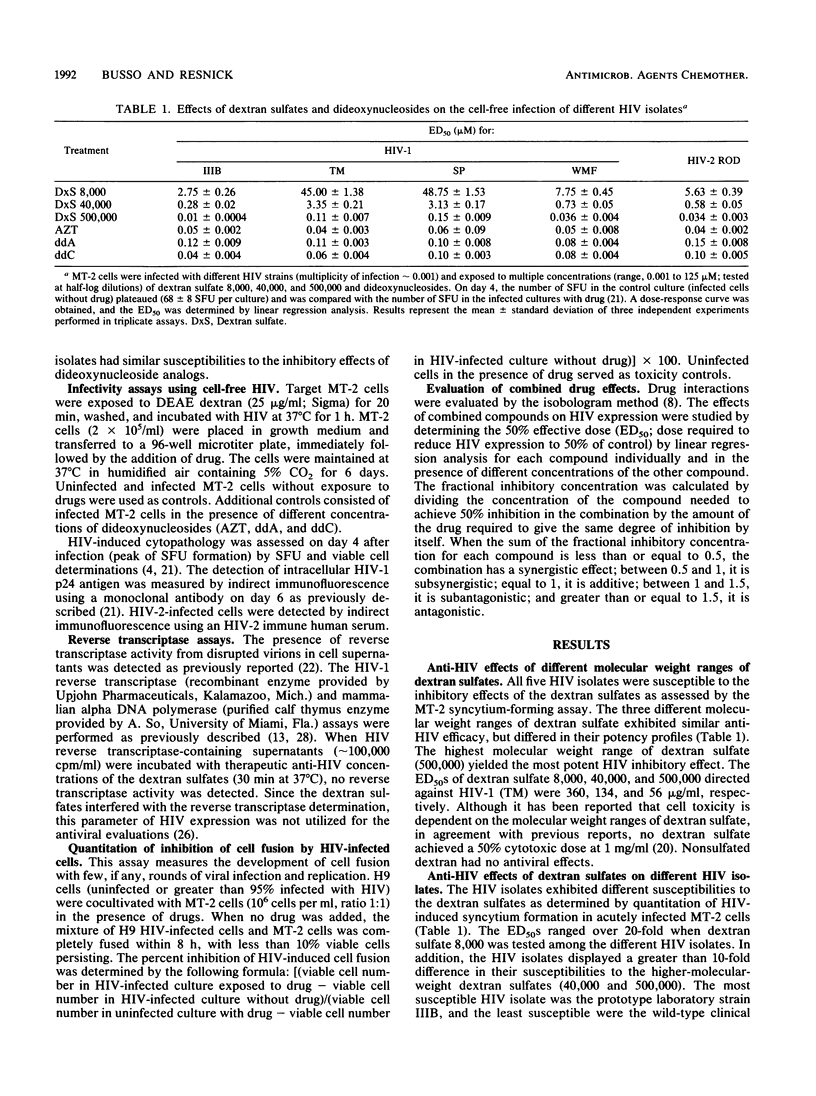
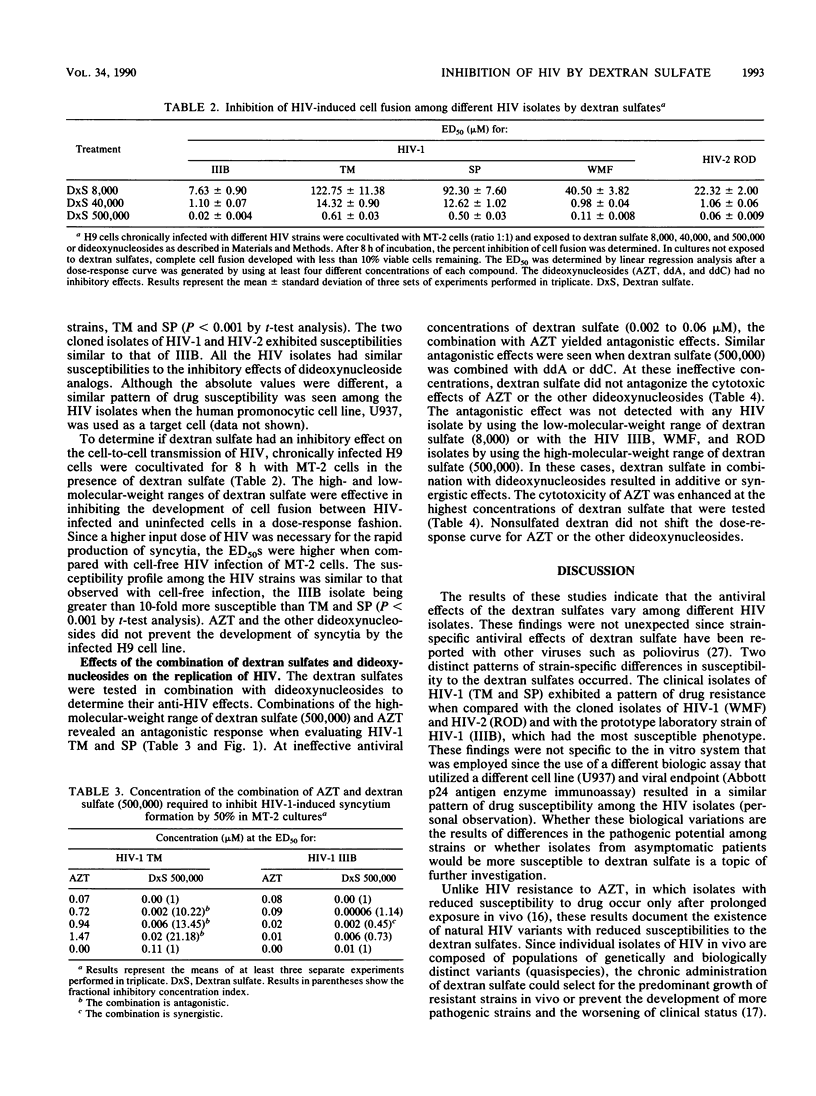
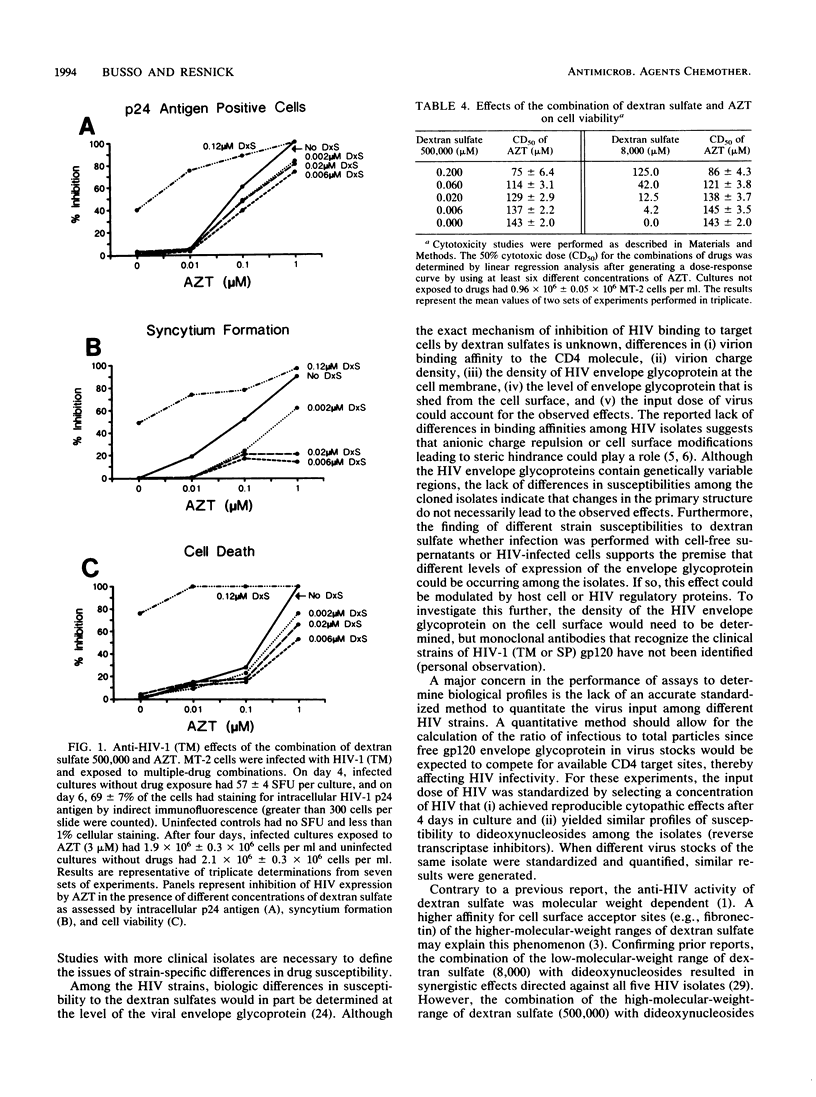
The effects of three molecular weight ranges of dextran sulfate on five different human immunodeficiency virus (HIV) isolates (from patients with acquired immunodeficiency syndrome), alone and in combination with dideoxynucleosides, were investigated in vitro. The higher the molecular weight range of dextran sulfate, the more potent the activity as assessed by a quantitative syncytium formation assay. Although all five HIV isolates had similar susceptibilities to the inhibitory effects of dideoxynucleosides, the two clinical isolates of HIV (HIV type 1 [HIV-1] TM and SP) exhibited a pattern of reduced susceptibility to dextran sulfate when compared with the two cloned isolates (HIV-1 WMF and HIV-2 ROD) and a prototype laboratory strain (HIV-1 IIIB). In combination with dideoxynucleosides, the high-molecular-weight range of dextran sulfate (500,000) resulted in an antagonistic response directed against the two clinical isolates of HIV (HIV-1 TM and SP) when the antiviral concentrations of dextran sulfate were in the ineffective range. Additive or synergistic effects were seen with the other three HIV isolates and all five HIV isolates when the low-molecular-weight range of dextran sulfate (8,000) was used. The results of these studies raise issues on the impact of drug-resistant strains on disease progression and the use of dextran sulfate in combination with nucleoside analogs for the clinical management of HIV disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba M., Pauwels R., Balzarini J., Arnout J., Desmyter J., De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Lischner H. W. Activity of dextran sulfate and other polyanionic polysaccharides against human immunodeficiency virus. J Infect Dis. 1988 Nov;158(5):1084–1087. doi: 10.1093/infdis/158.5.1084. [DOI] [PubMed] [Google Scholar]
- Busso M., Mian A. M., Hahn E. F., Resnick L. Nucleotide dimers suppress HIV expression in vitro. AIDS Res Hum Retroviruses. 1988 Dec;4(6):449–455. doi: 10.1089/aid.1988.4.449. [DOI] [PubMed] [Google Scholar]
- Byrn R. A., Sekigawa I., Chamow S. M., Johnson J. S., Gregory T. J., Capon D. J., Groopman J. E. Characterization of in vitro inhibition of human immunodeficiency virus by purified recombinant CD4. J Virol. 1989 Oct;63(10):4370–4375. doi: 10.1128/jvi.63.10.4370-4375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bârzu T., Van Rijn J. L., Petitou M., Molho P., Tobelem G., Caen J. P. Endothelial binding sites for heparin. Specificity and role in heparin neutralization. Biochem J. 1986 Sep 15;238(3):847–854. doi: 10.1042/bj2380847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Looney D., Rose A., Gallo R. C., Saag M. S., Shaw G. M., Hahn B. H., Wong-Staal F. Biologically diverse molecular variants within a single HIV-1 isolate. Nature. 1988 Aug 4;334(6181):444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- Gupta P., Balachandran R., Ho M., Enrico A., Rinaldo C. Cell-to-cell transmission of human immunodeficiency virus type 1 in the presence of azidothymidine and neutralizing antibody. J Virol. 1989 May;63(5):2361–2365. doi: 10.1128/jvi.63.5.2361-2365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Orentas R. J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989 Jun 2;244(4908):1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Johnson V. A., Barlow M. A., Chou T. C., Fisher R. A., Walker B. D., Hirsch M. S., Schooley R. T. Synergistic inhibition of human immunodeficiency virus type 1 (HIV-1) replication in vitro by recombinant soluble CD4 and 3'-azido-3'-deoxythymidine. J Infect Dis. 1989 May;159(5):837–844. doi: 10.1093/infdis/159.5.837. [DOI] [PubMed] [Google Scholar]
- Johnson V. A., Walker B. D., Barlow M. A., Paradis T. J., Chou T. C., Hirsch M. S. Synergistic inhibition of human immunodeficiency virus type 1 and type 2 replication in vitro by castanospermine and 3'-azido-3'-deoxythymidine. Antimicrob Agents Chemother. 1989 Jan;33(1):53–57. doi: 10.1128/aac.33.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Looney D. J., Kuno S., Ueno R., Wong-Staal F., Broder S. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science. 1988 Apr 29;240(4852):646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Kido Y., Kobayashi N., Motoki Y., Neushul M., Yamamoto N. Purification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob Agents Chemother. 1987 Oct;31(10):1524–1528. doi: 10.1128/aac.31.10.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H., Yoshida O., Tochikura T. S., Yoshida T., Mimura T., Kido Y., Motoki Y., Kaneko Y., Uryu T., Yamamoto N. Sulfation of polysaccharides generates potent and selective inhibitors of human immunodeficiency virus infection and replication in vitro. Jpn J Cancer Res. 1987 Nov;78(11):1164–1168. [PubMed] [Google Scholar]
- Nara P. L., Hatch W. C., Dunlop N. M., Robey W. G., Arthur L. O., Gonda M. A., Fischinger P. J. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987 Fall;3(3):283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Poiesz B. J., Ruscetti F. W., Gallo R. C. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1887–1891. doi: 10.1073/pnas.78.3.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick L., Markham P. D., Veren K., Salahuddin S. Z., Gallo R. C. In vitro suppression of HTLV-III/LAV infectivity by a combination of acyclovir and suramin. J Infect Dis. 1986 Dec;154(6):1027–1030. doi: 10.1093/infdis/154.6.1027. [DOI] [PubMed] [Google Scholar]
- Saag M. S., Hahn B. H., Gibbons J., Li Y., Parks E. S., Parks W. P., Shaw G. M. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988 Aug 4;334(6181):440–444. doi: 10.1038/334440a0. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., Cannon D. L., Arnold B. H., Martino-Saltzman D. Combinations of isoprinosine and 3'-azido-3'-deoxythymidine in lymphocytes infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1988 Dec;32(12):1784–1787. doi: 10.1128/aac.32.12.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., Eriksson B. F., Hughes S. H. Comparison of inhibitory activities of various antiretroviral agents against particle-derived and recombinant human immunodeficiency virus type 1 reverse transcriptases. Antimicrob Agents Chemother. 1989 Jan;33(1):115–117. doi: 10.1128/aac.33.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. II. Enhancement of plague formation and the detection of variants of poliovirus with dextran sulfate. Virology. 1962 Jul;17:499–501. doi: 10.1016/0042-6822(62)90148-4. [DOI] [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Ueno R., Kuno S. Dextran sulphate, a potent anti-HIV agent in vitro having synergism with zidovudine. Lancet. 1987 Jun 13;1(8546):1379–1379. doi: 10.1016/s0140-6736(87)90681-7. [DOI] [PubMed] [Google Scholar]
- WALTON K. W. Observations on the effects, of a series of dextran sulphates of varying molecular weight on the formed elements of the blood in vitro. Br J Pharmacol Chemother. 1953 Sep;8(3):340–347. doi: 10.1111/j.1476-5381.1953.tb00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


