Abstract
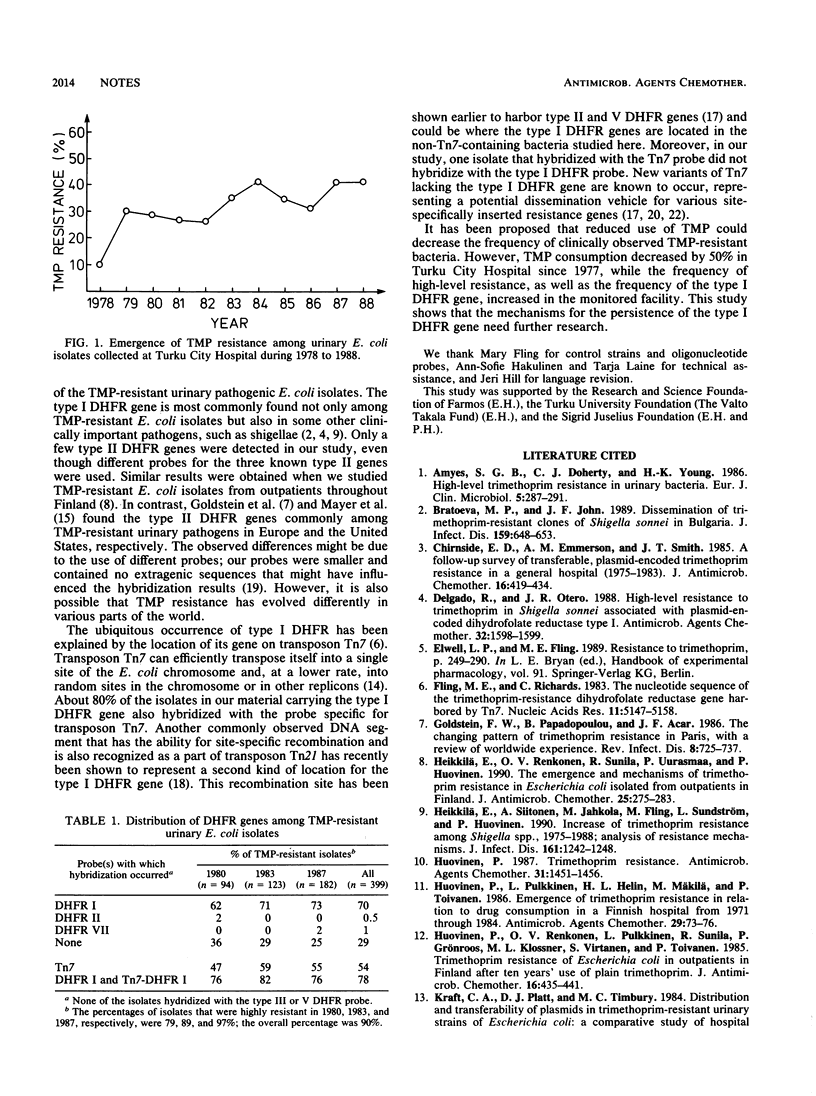
The frequency of trimethoprim resistance among Escherichia coli isolates from urine samples collected at Turku City Hospital, Turku, Finland, remained at 40% during 1984 to 1988. The proportion of highly resistant (MIC, greater than or equal to 1,024 micrograms/ml) isolates increased, however, and most of these harbored the type I dihydrofolate reductase gene. Only a few isolates possessed type II or VII genes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Doherty C. J., Young H. K. High-level trimethoprim resistance in urinary bacteria. Eur J Clin Microbiol. 1986 Jun;5(3):287–291. doi: 10.1007/BF02017783. [DOI] [PubMed] [Google Scholar]
- Bratoeva M. P., John J. F., Jr Dissemination of trimethoprim-resistant clones of Shigella sonnei in Bulgaria. J Infect Dis. 1989 Apr;159(4):648–653. doi: 10.1093/infdis/159.4.648. [DOI] [PubMed] [Google Scholar]
- Chirnside E. D., Emmerson A. M., Smith J. T. A follow-up survey of transferable, plasmid-encoded trimethoprim resistance in a general hospital (1975-1983). J Antimicrob Chemother. 1985 Oct;16(4):419–434. doi: 10.1093/jac/16.4.419. [DOI] [PubMed] [Google Scholar]
- Delgado R., Otero J. R. High-level resistance to trimethoprim in Shigella sonnei associated with plasmid-encoded dihydrofolate reductase type I. Antimicrob Agents Chemother. 1988 Oct;32(10):1598–1599. doi: 10.1128/aac.32.10.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling M. E., Richards C. The nucleotide sequence of the trimethoprim-resistant dihydrofolate reductase gene harbored by Tn7. Nucleic Acids Res. 1983 Aug 11;11(15):5147–5158. doi: 10.1093/nar/11.15.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein F. W., Papadopoulou B., Acar J. F. The changing pattern of trimethoprim resistance in Paris, with a review of worldwide experience. Rev Infect Dis. 1986 Sep-Oct;8(5):725–737. doi: 10.1093/clinids/8.5.725. [DOI] [PubMed] [Google Scholar]
- Heikkilä E., Renkonen O. V., Sunila R., Uurasmaa P., Huovinen P. The emergence and mechanisms of trimethoprim resistance in Escherichia coli isolated from outpatients in Finland. J Antimicrob Chemother. 1990 Feb;25(2):275–283. doi: 10.1093/jac/25.2.275. [DOI] [PubMed] [Google Scholar]
- Heikkilä E., Siitonen A., Jahkola M., Fling M., Sundström L., Huovinen P. Increase of trimethoprim resistance among Shigella species, 1975-1988: analysis of resistance mechanisms. J Infect Dis. 1990 Jun;161(6):1242–1248. doi: 10.1093/infdis/161.6.1242. [DOI] [PubMed] [Google Scholar]
- Huovinen P., Pulkkinen L., Helin H. L., Mäkilä M., Toivanen P. Emergence of trimethoprim resistance in relation to drug consumption in a Finnish hospital from 1971 through 1984. Antimicrob Agents Chemother. 1986 Jan;29(1):73–76. doi: 10.1128/aac.29.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovinen P., Renkonen O. V., Pulkkinen L., Sunila R., Grönroos P., Klossner M. L., Virtanen S., Toivanen P. Trimethoprim resistance of Escherichia coli in outpatients in Finland after ten years' use of plain trimethoprim. J Antimicrob Chemother. 1985 Oct;16(4):435–441. doi: 10.1093/jac/16.4.435. [DOI] [PubMed] [Google Scholar]
- Huovinen P. Trimethoprim resistance. Antimicrob Agents Chemother. 1987 Oct;31(10):1451–1456. doi: 10.1128/aac.31.10.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C. A., Platt D. J., Timbury M. C. Distribution and transferability of plasmids in trimethoprim-resistant urinary strains of Escherichia coli: a comparative study of hospital isolates. J Med Microbiol. 1984 Aug;18(1):95–105. doi: 10.1099/00222615-18-1-95. [DOI] [PubMed] [Google Scholar]
- Lichtenstein C., Brenner S. Unique insertion site of Tn7 in the E. coli chromosome. Nature. 1982 Jun 17;297(5867):601–603. doi: 10.1038/297601a0. [DOI] [PubMed] [Google Scholar]
- Mayer K. H., Fling M. E., Hopkins J. D., O'Brien T. F. Trimethoprim resistance in multiple genera of Enterobacteriaceae at a U.S. hospital: spread of the type II dihydrofolate reductase gene by a single plasmid. J Infect Dis. 1985 May;151(5):783–789. doi: 10.1093/infdis/151.5.783. [DOI] [PubMed] [Google Scholar]
- Sundström L., Rådström P., Swedberg G., Sköld O. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol Gen Genet. 1988 Aug;213(2-3):191–201. doi: 10.1007/BF00339581. [DOI] [PubMed] [Google Scholar]
- Sundström L., Sköld O. The dhfrI trimethoprim resistance gene of Tn7 can be found at specific sites in other genetic surroundings. Antimicrob Agents Chemother. 1990 Apr;34(4):642–650. doi: 10.1128/aac.34.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström L., Vinayagamoorthy T., Sköld O. Novel type of plasmid-borne resistance to trimethoprim. Antimicrob Agents Chemother. 1987 Jan;31(1):60–66. doi: 10.1128/aac.31.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze E., Brevet J., Tschäpe H. Relationships among the streptothricin resistance transposons Tn1825 and Tn1826 and the trimethoprim resistance transposon Tn7. Plasmid. 1987 Nov;18(3):246–249. doi: 10.1016/0147-619x(87)90067-9. [DOI] [PubMed] [Google Scholar]
- Towner K. J., Slack R. C. Effect of changing selection pressures on trimethoprim resistance in Enterobacteriaceae. Eur J Clin Microbiol. 1986 Oct;5(5):502–506. doi: 10.1007/BF02017691. [DOI] [PubMed] [Google Scholar]
- Wiedemann B., Meyer J. F., Zühlsdorf M. T. Insertions of resistance genes into Tn21-like transposons. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):85–92. doi: 10.1093/jac/18.supplement_c.85. [DOI] [PubMed] [Google Scholar]


