Abstract
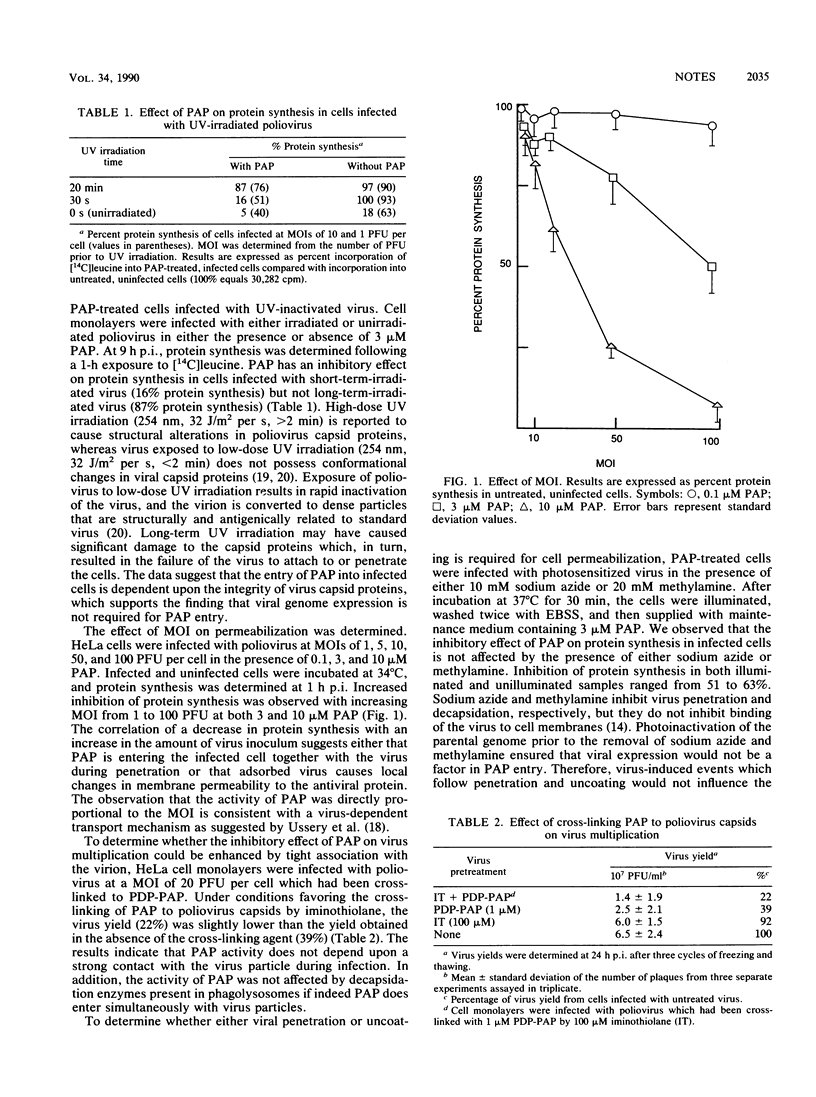
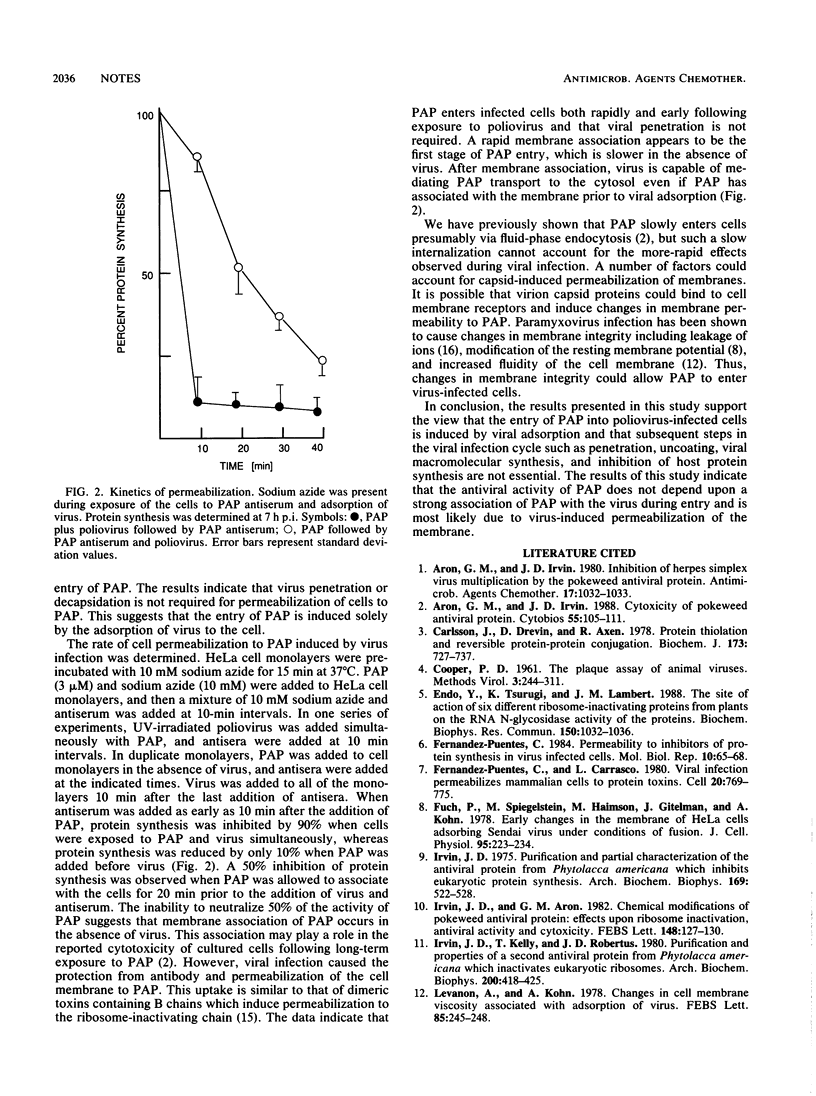
Infection of HeLa cells with poliovirus results in cell permeabilization to pokeweed antiviral protein. Cell permeabilization was dependent on the integrity of virus capsid proteins and directly proportional to the multiplicity of infection. This study demonstrates that virus adsorption is sufficient for the entry of pokeweed antiviral protein into poliovirus-infected cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Irvin J. D. Cytotoxicity of pokeweed antiviral protein. Cytobios. 1988;55(221):105–111. [PubMed] [Google Scholar]
- Aron G. M., Irvin J. D. Inhibition of herpes simplex virus multiplication by the pokeweed antiviral protein. Antimicrob Agents Chemother. 1980 Jun;17(6):1032–1033. doi: 10.1128/aac.17.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Tsurugi K., Lambert J. M. The site of action of six different ribosome-inactivating proteins from plants on eukaryotic ribosomes: the RNA N-glycosidase activity of the proteins. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1032–1036. doi: 10.1016/0006-291x(88)90733-4. [DOI] [PubMed] [Google Scholar]
- Fernández-Puentes C., Carrasco L. Viral infection permeabilizes mammalian cells to protein toxins. Cell. 1980 Jul;20(3):769–775. doi: 10.1016/0092-8674(80)90323-2. [DOI] [PubMed] [Google Scholar]
- Fernández-Puentes C. Permeability to inhibitors of protein synthesis in virus infected cells. Mol Biol Rep. 1984 Dec;10(2):65–68. doi: 10.1007/BF00776975. [DOI] [PubMed] [Google Scholar]
- Irvin J. D., Aron G. M. Chemical modifications of pokeweed antiviral protein: effects upon ribosome inactivation, antiviral activity and cytotoxicity. FEBS Lett. 1982 Nov 1;148(1):127–130. doi: 10.1016/0014-5793(82)81257-x. [DOI] [PubMed] [Google Scholar]
- Irvin J. D., Kelly T., Robertus J. D. Purification and properties of a second antiviral protein from Phytolacca americana which inactivates eukaryotic ribosomes. Arch Biochem Biophys. 1980 Apr 1;200(2):418–425. doi: 10.1016/0003-9861(80)90372-0. [DOI] [PubMed] [Google Scholar]
- Irvin J. D. Purification and partial characterization of the antiviral protein from Phytolacca americana which inhibits eukaryotic protein synthesis. Arch Biochem Biophys. 1975 Aug;169(2):522–528. doi: 10.1016/0003-9861(75)90195-2. [DOI] [PubMed] [Google Scholar]
- Levanon A., Kohn A. Changes in cell membrane microviscosity associated with adsorption of viruses. Differences between fusing and non-fusing viruses. FEBS Lett. 1978 Jan 15;85(2):245–248. doi: 10.1016/0014-5793(78)80465-7. [DOI] [PubMed] [Google Scholar]
- Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967 Apr;31(4):702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. Virally mediated membrane changes: inverse effects on transport and diffusion. Biochem J. 1974 Dec;144(3):593–595. doi: 10.1042/bj1440593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelstein P. F., Haimsohn M., Gitelman J., Kohn A. Early changes in the membrane of HeLa cells adsorbing Sendai virus under conditions of fusion. J Cell Physiol. 1978 May;95(2):223–233. doi: 10.1002/jcp.1040950212. [DOI] [PubMed] [Google Scholar]
- Tomlinson J. A., Walker V. M., Flewett T. H., Barclay G. R. The inhibition of infection by cucumber mosaic virus and influenza virus by extracts from Phytolacca americana. J Gen Virol. 1974 Feb;22(2):225–232. doi: 10.1099/0022-1317-22-2-225. [DOI] [PubMed] [Google Scholar]
- Ussery M. A., Irvin J. D., Hardesty B. Inhibition of poliovirus replication by a plant antiviral peptide. Ann N Y Acad Sci. 1977 Mar 4;284:431–440. doi: 10.1111/j.1749-6632.1977.tb21979.x. [DOI] [PubMed] [Google Scholar]
- Wetz K., Habermehl K. O. Specific cross-linking of capsid proteins to virus RNA by ultraviolet irradiation of poliovirus. J Gen Virol. 1982 Apr;59(Pt 2):397–401. doi: 10.1099/0022-1317-59-2-397. [DOI] [PubMed] [Google Scholar]
- Wetz K., Zeichhardt H., Willingmann P., Habermehl K. O. Dense particles and slow sedimenting particles produced by ultraviolet irradiation of poliovirus. J Gen Virol. 1983 Jun;64(Pt 6):1263–1275. doi: 10.1099/0022-1317-64-6-1263. [DOI] [PubMed] [Google Scholar]


