Abstract
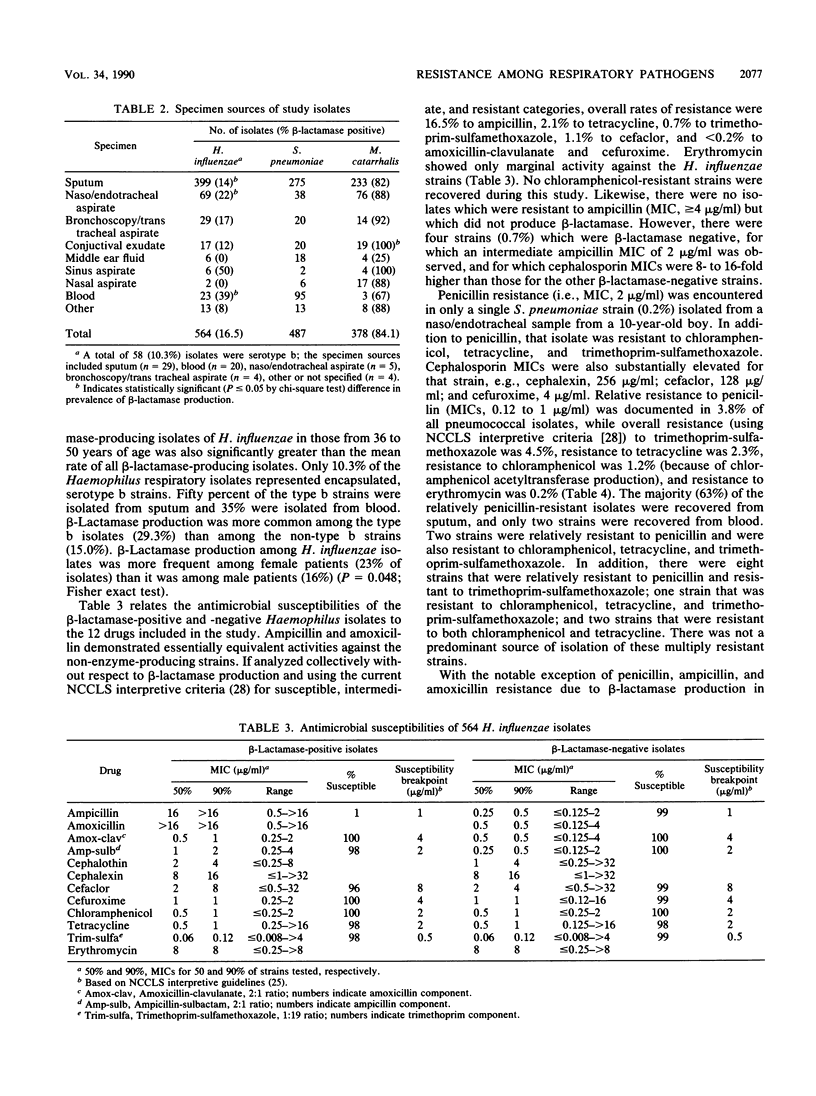
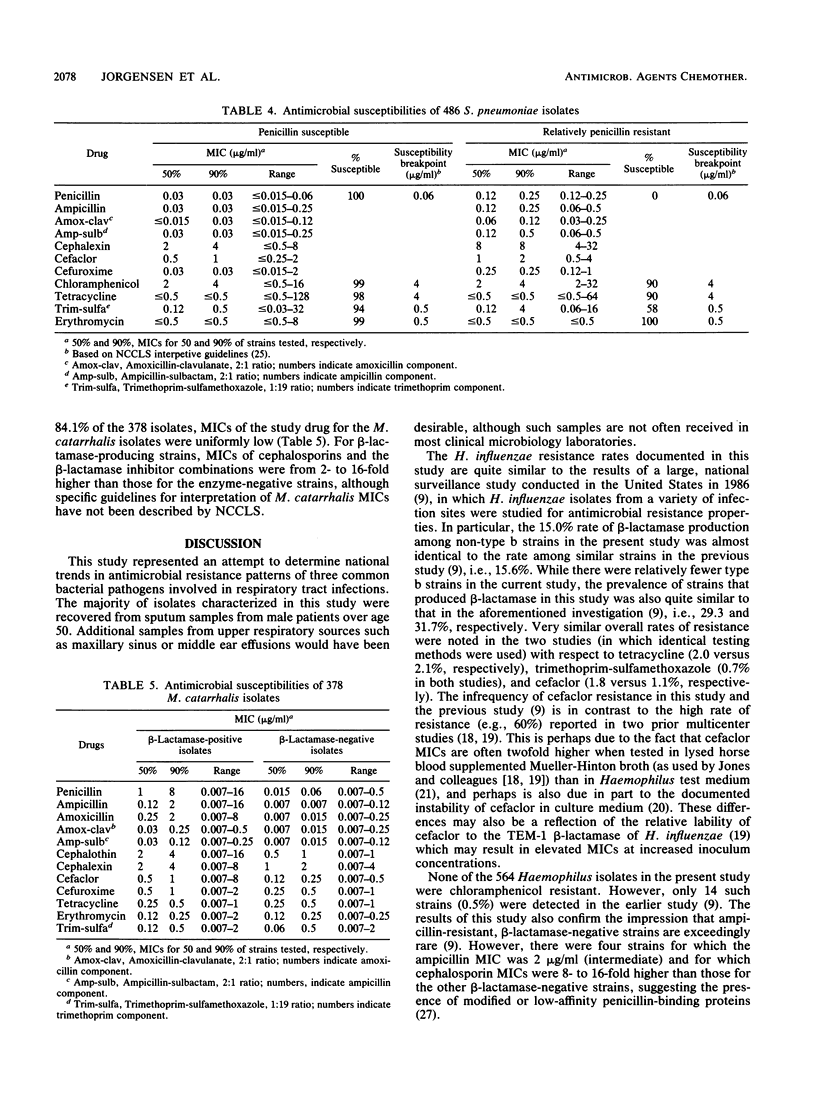
A national surveillance study was conducted to determine trends in antimicrobial resistance patterns among three common causes of community-acquired respiratory tract infections. Fifteen participating U.S. medical centers submitted clinically significant isolates of Haemophilus influenzae, Moraxella (Branhamella) catarrhalis, and Streptococcus pneumoniae to two central laboratories for testing with a group of 12 antimicrobial agents. The majority of isolates were recovered from adult males greater than 50 years old. Overall, 84.1% of 378 M. catarrhalis and 16.5% of 564 H. influenzae (29.5% of type b strains; 15.0% of non-type b strains) produced beta-lactamase and were thus resistant to penicillin, ampicillin, and amoxicillin. Resistance in H. influenzae to other agents was 2.1% to tetracycline, 0.7% to trimethoprim-sulfamethoxazole, 1.1% to cefaclor, and 0.2% to cefuroxime and amoxicillin-clavulanate, while the M. catarrhalis isolates yielded very low MICs of these latter drugs. As demonstrated in prior studies, erythromycin showed little activity against H. influenzae. Of 487 S. pneumoniae isolates, 1 (0.2%) was penicillin resistant, while 3.8% were relatively resistant to penicillin, 4.5% were resistant to trimethoprim-sulfamethoxazole, 2.3% were resistant to tetracycline, 1.2% were resistant to chloramphenicol, and 0.2% were resistant to erythromycin. Overall, the lowest resistance rates for these common bacterial respiratory pathogens were noted with amoxicillin-clavulanate, cefuroxime, and cefaclor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., McLeod D. T., Croughan M. J., Calder M. A. Antimicrobial susceptibility of Branhamella catarrhalis isolates from bronchopulmonary infections. Antimicrob Agents Chemother. 1984 Sep;26(3):424–425. doi: 10.1128/aac.26.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. A., Wallace R. J., Jr, Flanagan C. W., Wilson R. W., Luman J. I., Redditt S. D. Tetracycline and erythromycin resistance among clinical isolates of Branhamella catarrhalis. Antimicrob Agents Chemother. 1989 Sep;33(9):1631–1633. doi: 10.1128/aac.33.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder M. A., Croughan M. J., McLeod D. T., Ahmad F. The incidence and antibiotic susceptibility of Branhamella catarrhalis in respiratory infections. Drugs. 1986;31 (Suppl 3):11–16. doi: 10.2165/00003495-198600313-00005. [DOI] [PubMed] [Google Scholar]
- Calhoun K. H., Norris W. B., Hokanson J. A., Stiernberg C. M., Quinn F. B. Bacteriology of middle ear effusions. South Med J. 1988 Mar;81(3):332–336. doi: 10.1097/00007611-198803000-00012. [DOI] [PubMed] [Google Scholar]
- Dang-Van A., Tiraby G., Acar J. F., Shaw W. V., Bouanchaud D. H. Chloramphenicol resistance in Streptococcus pneumoniae: enzymatic acetylation and possible plasmid linkage. Antimicrob Agents Chemother. 1978 Apr;13(4):577–583. doi: 10.1128/aac.13.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V. Branhamella catarrhalis--an emerging human pathogen. Diagn Microbiol Infect Dis. 1986 Mar;4(3):191–201. doi: 10.1016/0732-8893(86)90098-2. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Jones R. N. Antimicrobial susceptibility testing of Haemophilus influenzae, Branhamella catarrhalis, and Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1988 Dec;32(12):1747–1753. doi: 10.1128/aac.32.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Jorgensen J. H., Thornsberry C., Preston D. A. Prevalence of antimicrobial resistance among clinical isolates of Haemophilus influenzae: a collaborative study. Diagn Microbiol Infect Dis. 1986 Feb;4(2):95–107. doi: 10.1016/0732-8893(86)90143-4. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Jorgensen J. H., Thornsberry C., Preston D. A., Tubert T., Redding J. S., Maher L. A. National collaborative study of the prevalence of antimicrobial resistance among clinical isolates of Haemophilus influenzae. Antimicrob Agents Chemother. 1988 Feb;32(2):180–185. doi: 10.1128/aac.32.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Tubert T. A. In vitro activities of 39 antimicrobial agents for Branhamella catarrhalis and comparison of results with different quantitative susceptibility test methods. Antimicrob Agents Chemother. 1988 Feb;32(2):259–261. doi: 10.1128/aac.32.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager H., Verghese A., Alvarez S., Berk S. L. Branhamella catarrhalis respiratory infections. Rev Infect Dis. 1987 Nov-Dec;9(6):1140–1149. doi: 10.1093/clinids/9.6.1140. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Gilligan P. H., Wait K., Goff D. A. Nasopharyngeal carriage of antibiotic-resistant pneumococci by children in group day care. J Infect Dis. 1988 Feb;157(2):256–263. doi: 10.1093/infdis/157.2.256. [DOI] [PubMed] [Google Scholar]
- Istre G. R., Humphreys J. T., Albrecht K. D., Thornsberry C., Swenson J. M., Hopkins R. S. Chloramphenicol and penicillin resistance in pneumococci isolated from blood and cerebrospinal fluid: a prevalence study in metropolitan Denver. J Clin Microbiol. 1983 Mar;17(3):472–475. doi: 10.1128/jcm.17.3.472-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istre G. R., Tarpay M., Anderson M., Pryor A., Welch D. Invasive disease due to Streptococcus pneumoniae in an area with a high rate of relative penicillin resistance. J Infect Dis. 1987 Nov;156(5):732–735. doi: 10.1093/infdis/156.5.732. [DOI] [PubMed] [Google Scholar]
- Jackson M. A., Shelton S., Nelson J. D., McCracken G. H., Jr Relatively penicillin-resistant pneumococcal infections in pediatric patients. Pediatr Infect Dis. 1984 Mar-Apr;3(2):129–132. doi: 10.1097/00006454-198403000-00010. [DOI] [PubMed] [Google Scholar]
- Jacobs M. R., Koornhof H. J., Robins-Browne R. M., Stevenson C. M., Vermaak Z. A., Freiman I., Miller G. B., Witcomb M. A., Isaäcson M., Ward J. I. Emergence of multiply resistant pneumococci. N Engl J Med. 1978 Oct 5;299(14):735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. BMY-28100, a new oral cephalosporin: antimicrobial activity against nearly 7,000 recent clinical isolates, comparative potency with other oral agents, and activity against beta-lactamase producing isolates. Diagn Microbiol Infect Dis. 1988 Jan;9(1):11–26. doi: 10.1016/0732-8893(88)90056-9. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Redding J. S., Maher L. A., Howell A. W. Improved medium for antimicrobial susceptibility testing of Haemophilus influenzae. J Clin Microbiol. 1987 Nov;25(11):2105–2113. doi: 10.1128/jcm.25.11.2105-2113.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Redding J. S., Maher L. A. Influence of storage and susceptibility test conditions on stability and activity of LY163892 and four other cephalosporins. Antimicrob Agents Chemother. 1988 Oct;32(10):1477–1480. doi: 10.1128/aac.32.10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousimies-Somer H. R., Savolainen S., Ylikoski J. S. Bacteriological findings of acute maxillary sinusitis in young adults. J Clin Microbiol. 1988 Oct;26(10):1919–1925. doi: 10.1128/jcm.26.10.1919-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman K. P., Koornhof H. J. Drug resistance patterns and serogroups or serotypes of pneumococcal isolates from cerebrospinal fluid or blood, 1979-1986. J Infect Dis. 1988 Nov;158(5):956–964. doi: 10.1093/infdis/158.5.956. [DOI] [PubMed] [Google Scholar]
- Latorre C., Juncosa T., Sanfeliu I. Antibiotic resistance and serotypes of 100 Streptococcus pneumoniae strains isolated in a children's hospital in Barcelona, Spain. Antimicrob Agents Chemother. 1985 Aug;28(2):357–359. doi: 10.1128/aac.28.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. W., Baker C. N., Thornsberry C. Relationship between in vitro susceptibility test results for chloramphenicol and production of chloramphenicol acetyltransferase by Haemophilus influenzae, Streptococcus pneumoniae, and Aerococcus species. J Clin Microbiol. 1988 Nov;26(11):2387–2390. doi: 10.1128/jcm.26.11.2387-2390.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Stull T. L., Rubens C. E., Mack K. D., Smith A. L. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984 Aug;26(2):235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpay M. M., Welch D. F., Salari H., Marks M. I. In vitro activity of antibiotics commonly used in the treatment of otitis media against Streptococcus pneumoniae isolates with different susceptibilities to penicillin. Antimicrob Agents Chemother. 1982 Jul;22(1):145–147. doi: 10.1128/aac.22.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]



